Apelin as a new therapeutic target for COVID-19 treatment
- PMID: 36200913
- PMCID: PMC9619586
- DOI: 10.1093/qjmed/hcac229
Apelin as a new therapeutic target for COVID-19 treatment
Abstract
Background: Apelin is an endogenous neuropeptide that binds to the G-protein-coupled receptor (APJ) and participates in a variety of physiological processes in the heart, lungs and other peripheral organs. Intriguingly, [Pyr1]-Apelin-13, a highly potent pyroglutamic form of apelin, has the potential to bind to and be degraded by angiotensin-converting enzyme 2 (ACE2). ACE2 is known to operate as a viral receptor in the early stages of severe acute respiratory coronavirus (SARS-CoV-2) infection.
Aim: This study aimed to determine if apelin protects against SARS-CoV-2 infection by inhibiting ACE2 binding to SARS-CoV-2 spike protein.
Design and methods: To determine whether [Pyr1]-Apelin-13 inhibits ACE2 binding to the SARS-CoV-2 spike protein (S protein), we performed a cell-to-cell fusion assay using ACE2-expressing cells and S protein-expressing cells and a pseudovirus-based inhibition assay. We then analyzed publicly available transcriptome data while focusing on the beneficial effects of apelin on the lungs.
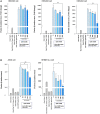
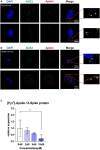
Results: We found that [Pyr1]-Apelin-13 inhibits cell-to-cell fusion mediated by ACE2 binding to the S protein. In this experiment, [Pyr1]-Apelin-13 protected human bronchial epithelial cells, infected with pseudo-typed lentivirus-producing S protein, against viral infection. In the presence of [Pyr1]-Apelin-13, the level of viral spike protein expression was also reduced in a concentration-dependent manner. Transcriptome analysis revealed that apelin may control inflammatory responses to viral infection by inhibiting the nuclear factor kappa B pathway.
Conclusion: Apelin is a potential therapeutic candidate against SARS-CoV-2 infection.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Association of Physicians.
Figures


Similar articles
-
Abnormal apelin-ACE2 and SGLT2 signaling contribute to adverse cardiorenal injury in patients with COVID-19.Int J Cardiol. 2021 Aug 1;336:123-129. doi: 10.1016/j.ijcard.2021.05.029. Epub 2021 May 15. Int J Cardiol. 2021. PMID: 34000358 Free PMC article.
-
Inhibiting the Deubiquitinase UCHL1 Reduces SARS-CoV-2 Viral Uptake by ACE2.Am J Respir Cell Mol Biol. 2023 May;68(5):566-576. doi: 10.1165/rcmb.2022-0331OC. Am J Respir Cell Mol Biol. 2023. PMID: 36730646 Free PMC article.
-
Single-cell analysis of SARS-CoV-2 receptor ACE2 and spike protein priming expression of proteases in the human heart.Cardiovasc Res. 2020 Aug 1;116(10):1733-1741. doi: 10.1093/cvr/cvaa191. Cardiovasc Res. 2020. PMID: 32638018 Free PMC article.
-
Angiotensin-Converting Enzyme 2 (ACE2) in the Pathogenesis of ARDS in COVID-19.Front Immunol. 2021 Dec 22;12:732690. doi: 10.3389/fimmu.2021.732690. eCollection 2021. Front Immunol. 2021. PMID: 35003058 Free PMC article. Review.
-
Regulation of Angiotensin-Converting Enzyme 2: A Potential Target to Prevent COVID-19?Front Endocrinol (Lausanne). 2021 Oct 22;12:725967. doi: 10.3389/fendo.2021.725967. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34745001 Free PMC article. Review.
Cited by
-
Analysis of Biomarker Levels in Nasopharyngeal Swabs, Serum, and Saliva Across Different Health Conditions.Life (Basel). 2025 Feb 19;15(2):324. doi: 10.3390/life15020324. Life (Basel). 2025. PMID: 40003732 Free PMC article.
-
Exploring the Network between Adipocytokines and Inflammatory Response in SARS-CoV-2 Infection: A Scoping Review.Nutrients. 2023 Aug 30;15(17):3806. doi: 10.3390/nu15173806. Nutrients. 2023. PMID: 37686837 Free PMC article.
-
Process-Specific Blood Biomarkers and Outcomes in COVID-19 Versus Non-COVID-19 ARDS (APEL-COVID Study): A Prospective, Observational Cohort Study.J Clin Med. 2024 Oct 4;13(19):5919. doi: 10.3390/jcm13195919. J Clin Med. 2024. PMID: 39407979 Free PMC article.
-
The development and application of pseudoviruses: assessment of SARS-CoV-2 pseudoviruses.PeerJ. 2023 Dec 6;11:e16234. doi: 10.7717/peerj.16234. eCollection 2023. PeerJ. 2023. PMID: 38077431 Free PMC article. Review.
-
Beneficial effects of Apelin-13 on metabolic diseases and exercise.Front Endocrinol (Lausanne). 2023 Nov 28;14:1285788. doi: 10.3389/fendo.2023.1285788. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 38089606 Free PMC article. Review.
References
-
- Maguire JJ, Kleinz MJ, Pitkin SL, Davenport APJH.. [Pyr1] apelin-13 identified as the predominant apelin isoform in the human heart: vasoactive mechanisms and inotropic action in disease. Hypertension 2009; 54:598–604. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

