Aberrant phosphorylation inactivates Numb in breast cancer causing expansion of the stem cell pool
- PMID: 36200956
- PMCID: PMC9545709
- DOI: 10.1083/jcb.202112001
Aberrant phosphorylation inactivates Numb in breast cancer causing expansion of the stem cell pool
Abstract
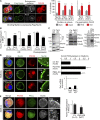
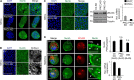
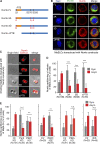
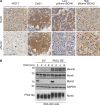
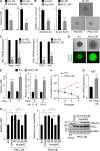
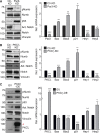
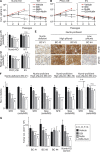
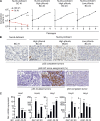
Asymmetric cell division is a key tumor suppressor mechanism that prevents the uncontrolled expansion of the stem cell (SC) compartment by generating daughter cells with alternative fates: one retains SC identity and enters quiescence and the other becomes a rapidly proliferating and differentiating progenitor. A critical player in this process is Numb, which partitions asymmetrically at SC mitosis and inflicts different proliferative and differentiative fates in the two daughters. Here, we show that asymmetric Numb partitioning per se is insufficient for the proper control of mammary SC dynamics, with differential phosphorylation and functional inactivation of Numb in the two progeny also required. The asymmetric phosphorylation/inactivation of Numb in the progenitor is mediated by the atypical PKCζ isoform. This mechanism is subverted in breast cancer via aberrant activation of PKCs that phosphorylate Numb in both progenies, leading to symmetric division and expansion of the cancer SC compartment, associated with aggressive disease. Thus, Numb phosphorylation represents a target for breast cancer therapy.
© 2022 Filippone et al.
Figures






References
-
- Albert, V., Piendl G., Yousseff D., Lammert H., Hummel M., Ortmann O., Jagla W., Gaumann A., Wege A.K., and Brockhoff G.. 2022. Protein kinase C targeting of luminal (T-47D), luminal/HER2-positive (BT474), and triple negative (HCC1806) breast cancer cells in-vitro with AEB071 (Sotrastaurin) is efficient but mediated by subtype specific molecular effects. Arch. Gynecol. Obstet. 306:1197–1210. 10.1007/s00404-022-06434-2 - DOI - PMC - PubMed
-
- Alsner, J., Jensen V., Kyndi M., Offersen B.V., Vu P., Borresen-Dale A.L., and Overgaard J.. 2008. A comparison between p53 accumulation determined by immunohistochemistry and TP53 mutations as prognostic variables in tumours from breast cancer patients. Acta Oncol. 47:600–607. 10.1080/02841860802047411 - DOI - PubMed
-
- Bellaiche, Y., Radovic A., Woods D.F., Hough C.D., Parmentier M.L., O’Kane C.J., Bryant P.J., and Schweisguth F.. 2001. The partner of inscuteable/Discs-large complex is required to establish planar polarity during asymmetric cell division in Drosophila. Cell. 106:355–366. 10.1016/s0092-8674(01)00444-5 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

