Mice with R2509C-RYR1 mutation exhibit dysfunctional Ca2+ dynamics in primary skeletal myocytes
- PMID: 36200983
- PMCID: PMC9546722
- DOI: 10.1085/jgp.202213136
Mice with R2509C-RYR1 mutation exhibit dysfunctional Ca2+ dynamics in primary skeletal myocytes
Abstract
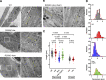
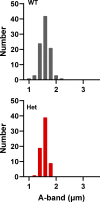
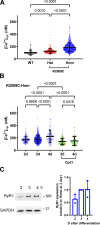
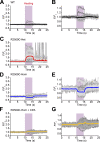
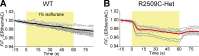
Type 1 ryanodine receptor (RYR1) is a Ca2+ release channel in the sarcoplasmic reticulum (SR) of the skeletal muscle and plays a critical role in excitation-contraction coupling. Mutations in RYR1 cause severe muscle diseases, such as malignant hyperthermia, a disorder of Ca2+-induced Ca2+ release (CICR) through RYR1 from the SR. We recently reported that volatile anesthetics induce malignant hyperthermia (MH)-like episodes through enhanced CICR in heterozygous R2509C-RYR1 mice. However, the characterization of Ca2+ dynamics has yet to be investigated in skeletal muscle cells from homozygous mice because these animals die in utero. In the present study, we generated primary cultured skeletal myocytes from R2509C-RYR1 mice. No differences in cellular morphology were detected between wild type (WT) and mutant myocytes. Spontaneous Ca2+ transients and cellular contractions occurred in WT and heterozygous myocytes, but not in homozygous myocytes. Electron microscopic observation revealed that the sarcomere length was shortened to ∼1.7 µm in homozygous myocytes, as compared to ∼2.2 and ∼2.3 µm in WT and heterozygous myocytes, respectively. Consistently, the resting intracellular Ca2+ concentration was higher in homozygous myocytes than in WT or heterozygous myocytes, which may be coupled with a reduced Ca2+ concentration in the SR. Finally, using infrared laser-based microheating, we found that heterozygous myocytes showed larger heat-induced Ca2+ transients than WT myocytes. Our findings suggest that the R2509C mutation in RYR1 causes dysfunctional Ca2+ dynamics in a mutant-gene dose-dependent manner in the skeletal muscles, in turn provoking MH-like episodes and embryonic lethality in heterozygous and homozygous mice, respectively.
© 2022 Tsuboi et al.
Figures








References
-
- Boncompagni, S., Rossi A.E., Micaroni M., Hamilton S.L., Dirksen R.T., Franzini-Armstrong C., and Protasi F.. 2009. Characterization and temporal development of cores in a mouse model of malignant hyperthermia. Proc. Natl. Acad. Sci. USA. 106:21996–22001. 10.1073/pnas.0911496106 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

