Spinal cord from body donors is suitable for multicolor immunofluorescence
- PMID: 36201037
- PMCID: PMC9899749
- DOI: 10.1007/s00418-022-02154-5
Spinal cord from body donors is suitable for multicolor immunofluorescence
Abstract
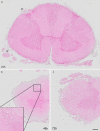
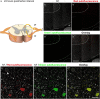
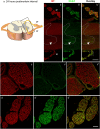
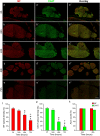
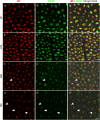
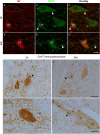
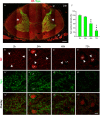
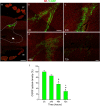
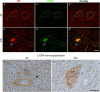
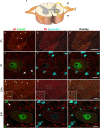
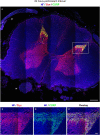
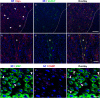
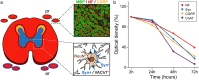
Immunohistochemistry is a powerful tool for studying neuronal tissue from humans at the molecular level. Obtaining fresh neuronal tissue from human organ donors is difficult and sometimes impossible. In anatomical body donations, neuronal tissue is dedicated to research purposes and because of its easier availability, it may be an alternative source for research. In this study, we harvested spinal cord from a single organ donor 2 h (h) postmortem and spinal cord from body donors 24, 48, and 72 h postmortem and tested how long after death, valid multi-color immunofluorescence or horseradish peroxidase (HRP) immunohistochemistry is possible. We used general and specific neuronal markers and glial markers for immunolabeling experiments. Here we showed that it is possible to visualize molecularly different neuronal elements with high precision in the body donor spinal cord 24 h postmortem and the quality of the image data was comparable to those from the fresh organ donor spinal cord. High-contrast multicolor images of the 24-h spinal cords allowed accurate automated quantification of different neuronal elements in the same sample. Although there was antibody-specific signal reduction over postmortem intervals, the signal quality for most antibodies was acceptable at 48 h but no longer at 72 h postmortem. In conclusion, our study has defined a postmortem time window of more than 24 h during which valid immunohistochemical information can be obtained from the body donor spinal cord. Due to the easier availability, neuronal tissue from body donors is an alternative source for basic and clinical research.
Keywords: Horseradish peroxidase immunohistochemistry; Human spinal cord; Multicolor immunofluorescence; Neuronal markers; Postmortem interval.
© 2022. The Author(s).
Conflict of interest statement
All the authors declare that there are no conflicts of interest.
Figures
Similar articles
-
Intraspinal transplantation of embryonic spinal cord tissue in neonatal and adult rats.J Comp Neurol. 1986 May 15;247(3):275-96. doi: 10.1002/cne.902470302. J Comp Neurol. 1986. PMID: 3522658
-
Immunohistochemical localization of substance P in postmortem rat and human spinal cord.J Neuropathol Exp Neurol. 1983 Jan;42(1):99-105. doi: 10.1097/00005072-198301000-00009. J Neuropathol Exp Neurol. 1983. PMID: 6185645
-
High-Spatial-Resolution Three-dimensional Imaging of Human Spinal Cord and Column Anatomy with Postmortem X-ray Phase-Contrast Micro-CT.Radiology. 2021 Jan;298(1):135-146. doi: 10.1148/radiol.2020201622. Epub 2020 Oct 27. Radiology. 2021. PMID: 33107800
-
Isolation of neural stem cells from the spinal cords of low temperature preserved abortuses.J Neurosci Methods. 2006 Oct 15;157(1):64-70. doi: 10.1016/j.jneumeth.2006.03.025. Epub 2006 May 8. J Neurosci Methods. 2006. PMID: 16682082
-
Mn (III) tetrakis (4-benzoic acid) porphyrin protects against neuronal and glial oxidative stress and death after spinal cord injury.CNS Neurol Disord Drug Targets. 2012 Sep;11(6):774-90. doi: 10.2174/187152712803581056. CNS Neurol Disord Drug Targets. 2012. PMID: 22483303 Free PMC article.
Cited by
-
Distal Nerve Transfers in High Peroneal Nerve Lesions: An Anatomical Feasibility Study.J Pers Med. 2023 Feb 16;13(2):344. doi: 10.3390/jpm13020344. J Pers Med. 2023. PMID: 36836578 Free PMC article.
-
Peripheral cranio-spinal nerve communication for trapezius muscle control using axonal profiling through immunostaining.Sci Rep. 2024 Oct 25;14(1):25266. doi: 10.1038/s41598-024-76645-x. Sci Rep. 2024. PMID: 39448752 Free PMC article.
References
-
- Almulhim AM, Menezes RG. Evaluation of Postmortem changes. Treasure Island (FL): StatPearls; 2022. - PubMed
-
- Blasco A, Gras S, Modol-Caballero G, Tarabal O, Casanovas A, Piedrafita L, et al. Motoneuron deafferentation and gliosis occur in association with neuromuscular regressive changes during ageing in mice. J Cachexia Sarcopenia Muscle. 2020;11(6):1628–1660. doi: 10.1002/jcsm.12599. - DOI - PMC - PubMed
-
- Blumer R, Maurer-Gesek B, Gesslbauer B, Blumer M, Pechriggl E, Davis-Lopez de Carrizosa MA, et al. Palisade endings are a constant feature in the extraocular muscles of frontal-eyed, but not lateral-eyed. Animals Invest Ophthalmol vis Sci. 2016;57(2):320–331. doi: 10.1167/iovs.15-18716. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

