Design, Synthesis, and Biochemical and Biological Evaluation of Novel 7-Deazapurine Cyclic Dinucleotide Analogues as STING Receptor Agonists
- PMID: 36201304
- PMCID: PMC9620234
- DOI: 10.1021/acs.jmedchem.2c01305
Design, Synthesis, and Biochemical and Biological Evaluation of Novel 7-Deazapurine Cyclic Dinucleotide Analogues as STING Receptor Agonists
Abstract
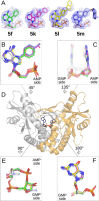
Cyclic dinucleotides (CDNs) are second messengers that activate stimulator of interferon genes (STING). The cGAS-STING pathway plays a promising role in cancer immunotherapy. Here, we describe the synthesis of CDNs containing 7-substituted 7-deazapurine moiety. We used mouse cyclic GMP-AMP synthase and bacterial dinucleotide synthases for the enzymatic synthesis of CDNs. Alternatively, 7-(het)aryl 7-deazapurine CDNs were prepared by Suzuki-Miyaura cross-couplings. New CDNs were tested in biochemical and cell-based assays for their affinity to human STING. Eight CDNs showed better activity than 2'3'-cGAMP, the natural ligand of STING. The effect on cytokine and chemokine induction was also evaluated. The best activities were observed for CDNs bearing large aromatic substituents that point above the CDN molecule. We solved four X-ray structures of complexes of new CDNs with human STING. We observed π-π stacking interactions between the aromatic substituents and Tyr240 that are involved in the stabilization of CDN-STING complexes.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







Similar articles
-
Crystal structures of porcine STINGCBD-CDN complexes reveal the mechanism of ligand recognition and discrimination of STING proteins.J Biol Chem. 2019 Jul 26;294(30):11420-11432. doi: 10.1074/jbc.RA119.007367. Epub 2019 Jun 5. J Biol Chem. 2019. PMID: 31167783 Free PMC article.
-
Design, Synthesis and Biological Evaluation of (2',5' and 3'5'-Linked) cGAMP Analogs that Activate Stimulator of Interferon Genes (STING).Molecules. 2020 Nov 12;25(22):5285. doi: 10.3390/molecules25225285. Molecules. 2020. PMID: 33198423 Free PMC article.
-
2',4'-LNA-Functionalized 5'-S-Phosphorothioester CDNs as STING Agonists.Chembiochem. 2024 Jul 2;25(13):e202400321. doi: 10.1002/cbic.202400321. Epub 2024 Jun 21. Chembiochem. 2024. PMID: 38720428
-
Novel Modifications and Delivery Modes of Cyclic Dinucleotides for STING Activation in Cancer Treatment.Int J Nanomedicine. 2025 Jan 6;20:181-197. doi: 10.2147/IJN.S503780. eCollection 2025. Int J Nanomedicine. 2025. PMID: 39802380 Free PMC article. Review.
-
Agonists and Inhibitors of the cGAS-STING Pathway.Molecules. 2024 Jun 30;29(13):3121. doi: 10.3390/molecules29133121. Molecules. 2024. PMID: 38999073 Free PMC article. Review.
Cited by
-
Late-stage guanine C8-H alkylation of nucleosides, nucleotides, and oligonucleotides via photo-mediated Minisci reaction.Nat Commun. 2024 Mar 21;15(1):2549. doi: 10.1038/s41467-024-46671-4. Nat Commun. 2024. PMID: 38514662 Free PMC article.
-
Lipid-based nanoparticles as drug delivery systems for cancer immunotherapy.MedComm (2020). 2023 Aug 7;4(4):e339. doi: 10.1002/mco2.339. eCollection 2023 Aug. MedComm (2020). 2023. PMID: 37560754 Free PMC article. Review.
-
The cGAS-STING pathway: a therapeutic target in diabetes and its complications.Burns Trauma. 2024 Feb 2;12:tkad050. doi: 10.1093/burnst/tkad050. eCollection 2024. Burns Trauma. 2024. PMID: 38312740 Free PMC article. Review.
-
The battle between the innate immune cGAS-STING signaling pathway and human herpesvirus infection.Front Immunol. 2023 Aug 2;14:1235590. doi: 10.3389/fimmu.2023.1235590. eCollection 2023. Front Immunol. 2023. PMID: 37600809 Free PMC article. Review.
-
Synthesis and Evaluation of Diguanosine Cap Analogs Modified at the C8-Position by Suzuki-Miyaura Cross-Coupling: Discovery of 7-Methylguanosine-Based Molecular Rotors.J Org Chem. 2023 Jun 2;88(11):6827-6846. doi: 10.1021/acs.joc.3c00126. Epub 2023 May 20. J Org Chem. 2023. PMID: 37209102 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Chemical Information
Research Materials
Miscellaneous

