Stereo- and Site-Selective Crotylation of Alcohol Proelectrophiles via Ruthenium-Catalyzed Hydrogen Auto-Transfer Mediated by Methylallene and Butadiene
- PMID: 36201364
- PMCID: PMC9712268
- DOI: 10.1002/anie.202212814
Stereo- and Site-Selective Crotylation of Alcohol Proelectrophiles via Ruthenium-Catalyzed Hydrogen Auto-Transfer Mediated by Methylallene and Butadiene
Abstract
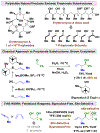
Iodide-bound ruthenium-JOSIPHOS complexes catalyze the redox-neutral C-C coupling of primary alcohols with methylallene (1,2-butadiene) or 1,3-butadiene to form products of anti-crotylation with good to excellent levels of diastereo- and enantioselectivity. Distinct from other methods, direct crotylation of primary alcohols in the presence of unprotected secondary alcohols is possible, enabling generation of spirastrellolide B (C9-C15) and leucascandrolide A (C9-C15) substructures in significantly fewer steps than previously possible.
Keywords: Alcohols; Crotylation; Dienes; Enantioselectivity; Ruthenium Catalysis.
© 2022 Wiley-VCH GmbH.
Figures



Similar articles
-
Ruthenium-Catalyzed Butadiene-Mediated Crotylation and Oxazaborolidine-Catalyzed Vinylogous Mukaiyama Aldol Reaction for The Synthesis of C1-C19 and C23-C35 of Neaumycin B.Angew Chem Int Ed Engl. 2022 Dec 23;61(52):e202214786. doi: 10.1002/anie.202214786. Epub 2022 Nov 23. Angew Chem Int Ed Engl. 2022. PMID: 36322115 Free PMC article.
-
Enantioselective C-H crotylation of primary alcohols via hydrohydroxyalkylation of butadiene.Science. 2012 Apr 20;336(6079):324-7. doi: 10.1126/science.1219274. Epub 2012 Mar 22. Science. 2012. PMID: 22442385 Free PMC article.
-
Ruthenium Catalyzed Diastereo- and Enantioselective Coupling of Propargyl Ethers with Alcohols: Siloxy-Crotylation via Hydride Shift Enabled Conversion of Alkynes to π-Allyls.J Am Chem Soc. 2015 Oct 14;137(40):13066-71. doi: 10.1021/jacs.5b08019. Epub 2015 Sep 29. J Am Chem Soc. 2015. PMID: 26418572 Free PMC article.
-
Ruthenium(0)-Catalyzed Cycloaddition of 1,2-Diols, Ketols, or Diones via Alcohol-Mediated Hydrogen Transfer.Angew Chem Int Ed Engl. 2018 Mar 12;57(12):3012-3021. doi: 10.1002/anie.201709916. Epub 2018 Feb 6. Angew Chem Int Ed Engl. 2018. PMID: 29068505 Free PMC article. Review.
-
Catalytic enantioselective C-H functionalization of alcohols by redox-triggered carbonyl addition: borrowing hydrogen, returning carbon.Angew Chem Int Ed Engl. 2014 Aug 25;53(35):9142-50. doi: 10.1002/anie.201403873. Epub 2014 Jul 23. Angew Chem Int Ed Engl. 2014. PMID: 25056771 Free PMC article. Review.
Cited by
-
Historical perspective on ruthenium-catalyzed hydrogen transfer and survey of enantioselective hydrogen auto-transfer processes for the conversion of lower alcohols to higher alcohols.Chem Sci. 2022 Oct 26;13(43):12625-12633. doi: 10.1039/d2sc05621f. eCollection 2022 Nov 9. Chem Sci. 2022. PMID: 36516346 Free PMC article. Review.
-
Dual Ruthenium-Catalyzed Alkene Isomerization-Hydrogen Auto-Transfer Unlocks Skipped Dienes as Pronucleophiles for Enantioselective Alcohol C-H Allylation.J Am Chem Soc. 2023 Apr 5:10.1021/jacs.3c00934. doi: 10.1021/jacs.3c00934. Online ahead of print. J Am Chem Soc. 2023. PMID: 37018070 Free PMC article.
-
Ruthenium-Catalyzed Butadiene-Mediated Crotylation and Oxazaborolidine-Catalyzed Vinylogous Mukaiyama Aldol Reaction for The Synthesis of C1-C19 and C23-C35 of Neaumycin B.Angew Chem Int Ed Engl. 2022 Dec 23;61(52):e202214786. doi: 10.1002/anie.202214786. Epub 2022 Nov 23. Angew Chem Int Ed Engl. 2022. PMID: 36322115 Free PMC article.
-
Chiral-at-Ruthenium-SEGPHOS Catalysts Display Diastereomer-Dependent Regioselectivity: Enantioselective Isoprene-Mediated Carbonyl tert-Prenylation via Halide Counterion Effects.J Am Chem Soc. 2023 Aug 23;145(33):18676-18683. doi: 10.1021/jacs.3c06734. Epub 2023 Aug 9. J Am Chem Soc. 2023. PMID: 37555765 Free PMC article.
-
Asymmetric Ruthenium-Catalyzed Carbonyl Allylations by Gaseous Allene via Hydrogen Auto-Transfer: 1° vs 2° Alcohol Dehydrogenation for Streamlined Polyketide Construction.ACS Catal. 2023 Feb 3;13(3):1662-1668. doi: 10.1021/acscatal.2c05425. Epub 2023 Jan 12. ACS Catal. 2023. PMID: 37869365 Free PMC article.
References
-
- Rohr J, Angew. Chem. Int. Ed 2000, 39, 2847–2849; - PubMed
- Angew. Chem 2000, 112, 2967–2969;
- Weissman KJ, Leadlay F, Nat. Rev. Microbiol 2005, 3, 925–936; - PubMed
- Rimando AM, Baerson SR, eds., Polyketides: Biosynthesis, Biological Activity, and Genetic Engineering (ACS Symp. Ser.,Vol. 955), American Chemical Society, Washington DC, 2007.
- Li JW-H, Vederas JC, Science 2009, 325, 161–165. - PubMed
-
- For selected reviews on catalytic enantioselective carbonyl allylation and crotylation, see: Denmark SE, Fu J, Chem. Rev 2003, 103, 2763–2794; - PubMed
- Hall DG, Synlett 2007, 1644–1655;
- Yus M, González-Gómez JC, Foubelo F, Chem. Rev 2011, 111, 7774–7854; - PubMed
- Huo H-X, Duvall JR, Huang M-Y, Hong R, Org. Chem. Front 2014, 1, 303–320;
- Spielmann K, Niel G, de Figueiredo RM, Campagne J-M, Chem. Soc. Rev 2018, 47, 1159–1173. - PubMed
-
- Carey JS, Laffan D, Thomson C, Williams MT, Org. Biomol. Chem 2006, 4, 2337–2347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

