The IL-4/IL-13 signaling axis promotes prostatic fibrosis
- PMID: 36201508
- PMCID: PMC9536598
- DOI: 10.1371/journal.pone.0275064
The IL-4/IL-13 signaling axis promotes prostatic fibrosis
Abstract
Background: Lower urinary tract symptoms (LUTS) are a costly and pervasive medical problem for millions of aging men. Recent studies have showed that peri-urethral tissue fibrosis is an untreated pathobiology contributing to LUTS. Fibrosis results from excessive extracellular matrix deposition which increases transition zone and peri-urethral tissue stiffness and compromises prostatic urethral flexibility and compliance, producing urinary obstructive symptoms. Inflammatory cells, including neutrophils, macrophages, and T-lymphocytes, secrete a medley of pro-fibrotic proteins into the prostatic microenvironment, including IFNγ, TNFα, CXC-type chemokines, and interleukins, all of which have been implicated in inflammation-mediated fibrosis. Among these, IL-4 and IL-13 are of particular interest because they share a common signaling axis that, as shown here for the first time, promotes the expression and maintenance of IL-4, IL-13, their cognate receptors, and ECM components by prostate fibroblasts, even in the absence of immune cells. Based on studies presented here, we hypothesize that the IL-4/IL-13 axis promotes prostate fibroblast activation to ECM-secreting cells.
Methods: N1 or SFT1 immortalized prostate stromal fibroblasts were cultured and treated, short- or long-term, with pro-fibrotic proteins including IL-4, IL-13, TGF-β, TNF-α, IFNγ, with or without prior pre-treatment with antagonists or inhibitors. Protein expression was assessed by immunohistochemistry, immunofluorescence, ELISA, immunoblot, or Sircoll assays. Transcript expression levels were determined by qRT-PCR. Intact cells were counted using WST assays.
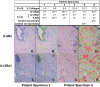
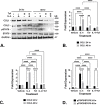
Results: IL-4Rα, IL-13Rα1, and collagen are concurrently up-regulated in human peri-urethral prostate tissues from men with LUTS. IL-4 and IL-13 induce their own expression as well as that of their cognate receptors, IL-4Rα and IL-13Rα1. Low concentrations of IL-4 or IL-13 act as cytokines to promote prostate fibroblast proliferation, but higher (>40ng/ml) concentrations repress cellular proliferation. Both IL-4 and IL-13 robustly and specifically promote collagen transcript and protein expression by prostate stromal fibroblasts in a JAK/STAT-dependent manner. Moreover, IL-4 and IL-13-mediated JAK/STAT signaling is coupled to activation of the IL-4Rα receptor.
Conclusions: Taken together, these studies show that IL-4 and IL-13 signal through the IL-4Rα receptor to activate JAK/STAT signaling, thereby promoting their own expression, that of their cognate receptors, and collagens. These finding suggest that the IL-4/IL-13 signaling axis is a powerful, but therapeutically targetable, pro-fibrotic mechanism in the lower urinary tract.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






Similar articles
-
CXC-type chemokines promote myofibroblast phenoconversion and prostatic fibrosis.PLoS One. 2012;7(11):e49278. doi: 10.1371/journal.pone.0049278. Epub 2012 Nov 16. PLoS One. 2012. PMID: 23173053 Free PMC article.
-
Endogenously expressed IL-13Rα2 attenuates IL-13-mediated responses but does not activate signaling in human lung fibroblasts.J Immunol. 2014 Jul 1;193(1):111-9. doi: 10.4049/jimmunol.1301761. Epub 2014 May 30. J Immunol. 2014. PMID: 24879793 Clinical Trial.
-
CXCL12/CXCR4 Axis Activation Mediates Prostate Myofibroblast Phenoconversion through Non-Canonical EGFR/MEK/ERK Signaling.PLoS One. 2016 Jul 19;11(7):e0159490. doi: 10.1371/journal.pone.0159490. eCollection 2016. PLoS One. 2016. PMID: 27434301 Free PMC article.
-
Chemokines and BPH/LUTS.Differentiation. 2011 Nov-Dec;82(4-5):253-60. doi: 10.1016/j.diff.2011.04.003. Epub 2011 May 19. Differentiation. 2011. PMID: 21600689 Free PMC article. Review.
-
The role of prostate inflammation and fibrosis in lower urinary tract symptoms.Am J Physiol Renal Physiol. 2016 Oct 1;311(4):F817-F821. doi: 10.1152/ajprenal.00602.2015. Epub 2016 Jul 20. Am J Physiol Renal Physiol. 2016. PMID: 27440781 Review.
Cited by
-
Aloe-emodin alleviates inflammatory bowel disease in mice by modulating intestinal microbiome homeostasis via the IL-4/IL-13 axis.Heliyon. 2024 Jul 22;10(15):e34932. doi: 10.1016/j.heliyon.2024.e34932. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39157379 Free PMC article.
-
Current research and future directions in non-malignant urologic research - proceedings of the annual CAIRIBU meeting.Am J Clin Exp Urol. 2022 Dec 25;10(6):449-461. eCollection 2022. Am J Clin Exp Urol. 2022. PMID: 36636691 Free PMC article.
-
Immunosuppressive tumor microenvironment and uterine fibroids: Role in collagen synthesis.Cytokine Growth Factor Rev. 2024 Feb;75:93-100. doi: 10.1016/j.cytogfr.2023.10.002. Epub 2023 Oct 6. Cytokine Growth Factor Rev. 2024. PMID: 37839993 Free PMC article. Review.
-
Fibroblasts and immune cells: at the crossroad of organ inflammation and fibrosis.Am J Physiol Heart Circ Physiol. 2024 Feb 1;326(2):H303-H316. doi: 10.1152/ajpheart.00545.2023. Epub 2023 Dec 1. Am J Physiol Heart Circ Physiol. 2024. PMID: 38038714 Free PMC article. Review.
-
The Role of IL-13 and IL-4 in Adipose Tissue Fibrosis.Int J Mol Sci. 2023 Mar 16;24(6):5672. doi: 10.3390/ijms24065672. Int J Mol Sci. 2023. PMID: 36982747 Free PMC article.
References
-
- Kupelian V, Wei JT, O’Leary MP, Kusek JW, Litman HJ, Link CL, et al.. Prevalence of lower urinary tract symptoms and effect on quality of life in a racially and ethnically diverse random sample: the Boston Area Community Health (BACH) Survey. Arch Intern Med. 2006;166(21):2381–7. Epub 2006/11/30. 166/21/2381 [pii] doi: 10.1001/archinte.166.21.2381 . - DOI - PubMed
-
- Strope SA, Yang L, Nepple KG, Andriole GL, Owens PL. Population based comparative effectiveness of transurethral resection of the prostate and laser therapy for benign prostatic hyperplasia. J Urol. 2012;187(4):1341–5. doi: 10.1016/j.juro.2011.11.102 ; PubMed Central PMCID: PMC3461307. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

