Therapeutic efficacy of rifalazil (KRM-1648) in a M. ulcerans-induced Buruli ulcer mouse model
- PMID: 36201529
- PMCID: PMC9536621
- DOI: 10.1371/journal.pone.0274742
Therapeutic efficacy of rifalazil (KRM-1648) in a M. ulcerans-induced Buruli ulcer mouse model
Abstract
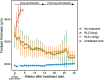
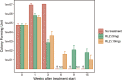
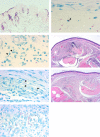
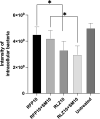
Buruli ulcer (BU) is a skin disease caused by Mycobacterium ulcerans infection that requires long-term antibiotic treatment and/or surgical excision. In this study, we investigated the therapeutic efficacy of the rifamycin derivative, rifalazil (RLZ) (also known as KRM-1648), in an advanced M. ulcerans infection model. Six-week-old female BALB/c mice were infected with 3.25 x 104 colony-forming units (CFU) of M. ulcerans subcutaneously into the bilateral hind footpads. At 33 days post-infection, when the footpads exhibited significant redness and swelling, mice were treated orally with 5 or 10 mg/kg of RLZ for up to 15 weeks. Mice were followed for an additional 15 weeks following treatment cessation. Untreated mice exhibited a progressive increase in footpad redness, swelling, and erosion over time, and all untreated mice reached to endpoint within 5-8 weeks post-bacterial injection. In the RLZ-treated mice, footpad redness and swelling and general condition improved or completely healed, and no recurrence occurred following treatment cessation. After 3 weeks of treatment, the CFU counts from the footpads of recovered RLZ-treated mice showed a 104 decrease compared with those of untreated mice. We observed a further reduction in CFU counts to the detection limit following 6 to 15 weeks of treatment, which did not increase 15 weeks after discontinuing the treatment. Histopathologically, bacteria in the treated mice became fragmented one week after RLZ-treatment. At the final point of the experiment, all the treated mice (5mg/kg/day; n = 6, 10mg/kg/day; n = 7) survived and had no signs of M. ulcerans infection. These results indicate that the rifamycin analogue, RLZ, is efficacious in the treatment of an advanced M. ulcerans infection mouse model.
Conflict of interest statement
No
Figures

References
-
- Buruli ulcer (Mycobacterium ulcerans infection). [cited 22 Jul 2020]. Available: https://www.who.int/news-room/fact-sheets/detail/buruli-ulcer-(mycobacte...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

