Sequestration of gut pathobionts in intraluminal casts, a mechanism to avoid dysregulated T cell activation by pathobionts
- PMID: 36201539
- PMCID: PMC9565271
- DOI: 10.1073/pnas.2209624119
Sequestration of gut pathobionts in intraluminal casts, a mechanism to avoid dysregulated T cell activation by pathobionts
Abstract
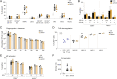
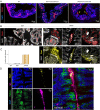
T cells that express the transcription factor RORγ, regulatory (Treg), or conventional (Th17) are strongly influenced by intestinal symbionts. In a genetic approach to identify mechanisms underlying this influence, we performed a screen for microbial genes implicated, in germfree mice monocolonized with Escherichia coli Nissle. The loss of capsule-synthesis genes impaired clonal expansion and differentiation of intestinal RORγ+ T cells. Mechanistic exploration revealed that the capsule-less mutants remained able to induce species-specific immunoglobulin A (IgA) and were highly IgA-coated. They could still trigger myeloid cells, and more effectively damaged epithelial cells in vitro. Unlike wild-type microbes, capsule-less mutants were mostly engulfed in intraluminal casts, large agglomerates composed of myeloid cells extravasated into the gut lumen. We speculate that sequestration in luminal casts of potentially harmful microbes, favored by IgA binding, reduces the immune system's actual exposure, preserving host-microbe equilibrium. The variable immunostimulation by microbes that has been charted in recent years may not solely be conditioned by triggering molecules or metabolites but also by physical limits to immune system exposure.
Keywords: CD4+ T cell; IgA; capsule; genetic screen; gut.
Conflict of interest statement
The authors declare no competing interest.
Figures





Similar articles
-
Developmental and cellular age direct conversion of CD4+ T cells into RORγ+ or Helios+ colon Treg cells.J Exp Med. 2020 Jan 6;217(1):e20190428. doi: 10.1084/jem.20190428. J Exp Med. 2020. PMID: 31685531 Free PMC article.
-
Microbial bile acid metabolites modulate gut RORγ+ regulatory T cell homeostasis.Nature. 2020 Jan;577(7790):410-415. doi: 10.1038/s41586-019-1865-0. Epub 2019 Dec 25. Nature. 2020. PMID: 31875848 Free PMC article.
-
An Immunologic Mode of Multigenerational Transmission Governs a Gut Treg Setpoint.Cell. 2020 Jun 11;181(6):1276-1290.e13. doi: 10.1016/j.cell.2020.04.030. Epub 2020 May 12. Cell. 2020. PMID: 32402238 Free PMC article.
-
Gut TFH and IgA: key players for regulation of bacterial communities and immune homeostasis.Immunol Cell Biol. 2014 Jan;92(1):49-56. doi: 10.1038/icb.2013.54. Epub 2013 Oct 8. Immunol Cell Biol. 2014. PMID: 24100385 Review.
-
Interactions of commensal gut microbes with subsets of B- and T-cells in the murine host.Vaccine. 2004 Feb 17;22(7):805-11. doi: 10.1016/j.vaccine.2003.11.022. Vaccine. 2004. PMID: 15040931 Review.
Cited by
-
The right educational environment: Oral tolerance in early life.Immunol Rev. 2024 Sep;326(1):17-34. doi: 10.1111/imr.13366. Epub 2024 Jul 13. Immunol Rev. 2024. PMID: 39001685 Review.
-
Regulatory T cells in the face of the intestinal microbiota.Nat Rev Immunol. 2023 Nov;23(11):749-762. doi: 10.1038/s41577-023-00890-w. Epub 2023 Jun 14. Nat Rev Immunol. 2023. PMID: 37316560 Review.
-
The role of the glycome in symbiotic host-microbe interactions.Glycobiology. 2023 Dec 30;33(12):1106-1116. doi: 10.1093/glycob/cwad073. Glycobiology. 2023. PMID: 37741057 Free PMC article. Review.
-
Homeostatic, repertoire and transcriptional relationships between colon T regulatory cell subsets.Proc Natl Acad Sci U S A. 2023 Dec 12;120(50):e2311566120. doi: 10.1073/pnas.2311566120. Epub 2023 Dec 8. Proc Natl Acad Sci U S A. 2023. PMID: 38064511 Free PMC article.
-
The role of gut microbiota in intestinal disease: from an oxidative stress perspective.Front Microbiol. 2024 Feb 14;15:1328324. doi: 10.3389/fmicb.2024.1328324. eCollection 2024. Front Microbiol. 2024. PMID: 38419631 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

