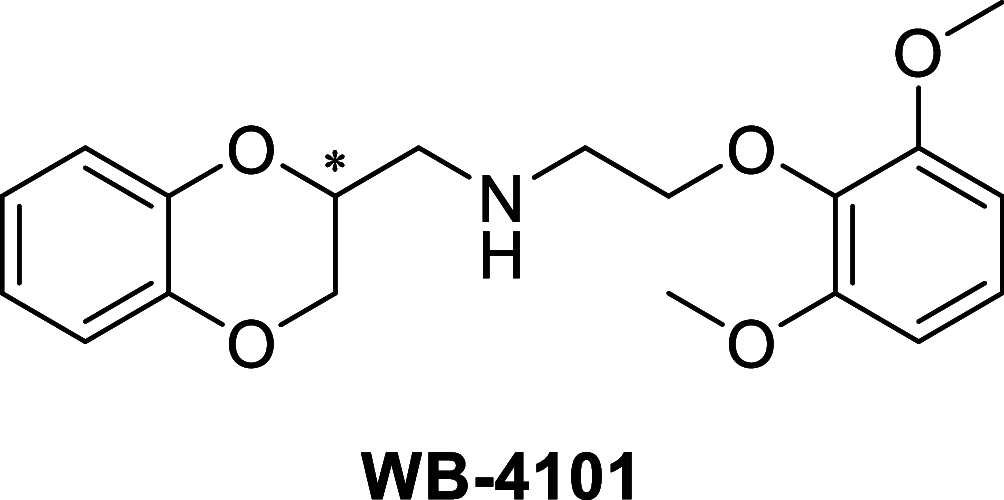
Discovery of a Potent and Highly Selective Dipeptidyl Peptidase IV and Carbonic Anhydrase Inhibitor as "Antidiabesity" Agents Based on Repurposing and Morphing of WB-4101
- PMID: 36201615
- PMCID: PMC9937538
- DOI: 10.1021/acs.jmedchem.2c01192
Discovery of a Potent and Highly Selective Dipeptidyl Peptidase IV and Carbonic Anhydrase Inhibitor as "Antidiabesity" Agents Based on Repurposing and Morphing of WB-4101
Abstract
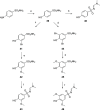
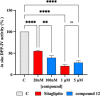
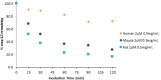
The management of patients with type 2 diabetes mellitus (T2DM) is shifting from cardio-centric to weight-centric or, even better, adipose-centric treatments. Considering the downsides of multidrug therapies and the relevance of dipeptidyl peptidase IV (DPP IV) and carbonic anhydrases (CAs II and V) in T2DM and in the weight loss, we report a new class of multitarget ligands targeting the mentioned enzymes. We started from the known α1-AR inhibitor WB-4101, which was progressively modified through a tailored morphing strategy to optimize the potency of DPP IV and CAs while losing the adrenergic activity. The obtained compound 12 shows a satisfactory DPP IV inhibition with a good selectivity CA profile (DPP IV IC50: 0.0490 μM; CA II Ki 0.2615 μM; CA VA Ki 0.0941 μM; CA VB Ki 0.0428 μM). Furthermore, its DPP IV inhibitory activity in Caco-2 and its acceptable pre-ADME/Tox profile indicate it as a lead compound in this novel class of multitarget ligands.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Chemical Information
Medical
Research Materials

