The antioxidant and oxidant properties of hydropersulfides (RSSH) and polysulfide species
- PMID: 36201912
- PMCID: PMC9535303
- DOI: 10.1016/j.redox.2022.102486
The antioxidant and oxidant properties of hydropersulfides (RSSH) and polysulfide species
Abstract
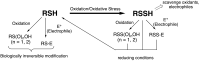
It has become apparent that hydrogen sulfide (H2S), hydropersulfides (RSSH) and other polysulfide species are all intimately linked biochemically. Indeed, at least some of the biological activity attributed to hydrogen sulfide (H2S) may actually be due to its conversion to RSSH and derived polysulfur species (and vice-versa). The unique chemistry associated with the hydropersulfide functional group (-SSH) predicts that it possesses possible protective properties that can help a cell contend with oxidative and/or electrophilic stress. However, since RSSH and polysulfides possess chemical properties akin to disulfides (RSSR), they can also be sources of oxidative/electrophilic stress/signaling as well. Herein are discussed the unique chemistry, possible biochemistry and the physiological implications of RSSH (and polysulfides), especially as it pertains to their putative cellular protection properties against a variety of stresses and/or as possible stressors/signaling agents themselves.
Keywords: Hydrogen sulfide; Hydropersulfides; Polysulfides; S-nitrosothiol.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare no conflicts of interest.
Figures


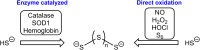
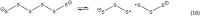

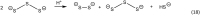

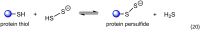
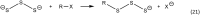

Similar articles
-
The chemistry of hydropersulfides (RSSH) as related to possible physiological functions.Arch Biochem Biophys. 2023 Jul 15;743:109659. doi: 10.1016/j.abb.2023.109659. Epub 2023 May 30. Arch Biochem Biophys. 2023. PMID: 37263465 Review.
-
Chemical Biology of Hydropersulfides and Related Species: Possible Roles in Cellular Protection and Redox Signaling.Antioxid Redox Signal. 2017 Oct 1;27(10):622-633. doi: 10.1089/ars.2017.7081. Epub 2017 Jun 13. Antioxid Redox Signal. 2017. PMID: 28398141 Review.
-
The Biological/Physiological Utility of Hydropersulfides (RSSH) and Related Species: What Is Old Is New Again.Antioxid Redox Signal. 2022 Feb;36(4-6):244-255. doi: 10.1089/ars.2021.0096. Epub 2021 Jun 21. Antioxid Redox Signal. 2022. PMID: 33985355 Review.
-
A comparison of the chemical biology of hydropersulfides (RSSH) with other protective biological antioxidants and nucleophiles.Nitric Oxide. 2021 Feb 1;107:46-57. doi: 10.1016/j.niox.2020.11.004. Epub 2020 Nov 28. Nitric Oxide. 2021. PMID: 33253886 Review.
-
Development of Hydropersulfide Donors to Study Their Chemical Biology.Antioxid Redox Signal. 2022 Feb;36(4-6):309-326. doi: 10.1089/ars.2021.0149. Epub 2021 Sep 22. Antioxid Redox Signal. 2022. PMID: 34278824 Review.
Cited by
-
Recent advances in sulfur biology and chemistry.Redox Biol. 2023 Jul;63:102716. doi: 10.1016/j.redox.2023.102716. Epub 2023 Apr 24. Redox Biol. 2023. PMID: 37127439 Free PMC article. No abstract available.
-
SOD1 is an essential H2S detoxifying enzyme.Proc Natl Acad Sci U S A. 2023 Jan 17;120(3):e2205044120. doi: 10.1073/pnas.2205044120. Epub 2023 Jan 11. Proc Natl Acad Sci U S A. 2023. PMID: 36630448 Free PMC article.
-
Hydropersulfides (RSSH) attenuate doxorubicin-induced cardiotoxicity while boosting its anticancer action.Redox Biol. 2023 Apr;60:102625. doi: 10.1016/j.redox.2023.102625. Epub 2023 Feb 4. Redox Biol. 2023. PMID: 36773545 Free PMC article.
-
Reactive Oxygen Species Signaling and Oxidative Stress: Transcriptional Regulation and Evolution.Antioxidants (Basel). 2024 Mar 1;13(3):312. doi: 10.3390/antiox13030312. Antioxidants (Basel). 2024. PMID: 38539845 Free PMC article. Review.
References
-
- Fukuto J.M., Carrington S.J., Tantillo D.J., Harrison J.G., Ignarro L.J., Freeman B.A., Chen A., Wink D.A. Small molecule signaling agents: the integrated chemistry and biochemistry of nitrogen oxides, oxides of carbon, dioxygen, hydrogen sulfide and their derived species. Chem. Res. Toxicol. 2012;25:769–793. - PMC - PubMed
-
- Fukuto J.M., Collins M.D. Interactive endogenous small molecule (gaseous) signaling: implications for teratogenesis. Curr. Pharmaceut. Des. 2007;13(29):2952–2978. - PubMed
-
- Bianco C.L., Akaike T., Ida T., Nagy P., Bogdandi V., Toscano J.P., Kumagai Y., Henderson C.F., Goddu R.N., Lin J., Fukuto J.M. The reaction of hydrogen sulfide with disulfides: formation of a stable trisulfide and implications for biological systems. Br. J. Pharmacol. 2019;176:671–683. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

