A case report of an 18-year-old receiving nebulized lidocaine for treatment of COVID-19 cough
- PMID: 36201924
- PMCID: PMC9515246
- DOI: 10.1016/j.hrtlng.2022.09.009
A case report of an 18-year-old receiving nebulized lidocaine for treatment of COVID-19 cough
Abstract
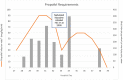
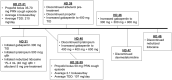
An 18-year-old girl presenting with respiratory and gastrointestinal symptoms was found to have COVID-19 pneumonia and severe acute respiratory distress syndrome (ARDS). She was transferred to our pediatric intensive care unit (PICU) for ongoing mechanical ventilation and initiation of venovenous extracorporeal membrane oxygenation (VV-ECMO) for management of progressive hypoxic respiratory failure. She developed a worsening cough with associated life-threatening desaturation events that impaired ECMO flow and required deep sedation. Despite multiple sedative agents, our patient continued to have frequent coughing episodes with associated tachycardia, hypertension, and hypoxemia. The PICU team started nebulized lidocaine 1% 4 mL (40 mg) every 6 hours with albuterol pretreatment, gabapentin, and scheduled ipratropium. Lidocaine levels were <1 mcg/mL throughout the treatment duration. Nebulized lidocaine was stopped after 18 days given improvement in coughing episode severity. Our patient is one of the first reports of an adolescent patient receiving nebulized lidocaine for COVID-19 associated cough. Administration of nebulized lidocaine was well tolerated in this patient without adverse effects and was associated with decreased sedation needs. Given the widespread impact of the COVID-19 pandemic and its sequelae in pediatric, adolescent, and adult patients, additional research is warranted to explore options for management of COVID-19 associated cough.
Keywords: Adolescent; COVID-19; Child; Cough; Extracorporeal membrane oxygenation; Lidocaine.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest None.
Figures
References
-
- Ryan NM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a randomised, double-blind, placebo-controlled trial. Lancet. 2012;380:1583–1589. - PubMed
-
- Vertigan AE, Kapela SL, Ryan NM, Birring SS, McElduff P, Gibson PG. Pregabalin and speech pathology combination therapy for refractory chronic cough: a randomized controlled trial. Chest. 2016;149:639–648. - PubMed
-
- Holmes PW, Barter CE, Pierce RJ. Chronic persistent cough: use of ipratropium bromide in undiagnosed cases following upper respiratory tract infection. Respir Med. 1992;86:425–429. - PubMed