Cortical lesions, central vein sign, and paramagnetic rim lesions in multiple sclerosis: Emerging machine learning techniques and future avenues
- PMID: 36201950
- PMCID: PMC9668629
- DOI: 10.1016/j.nicl.2022.103205
Cortical lesions, central vein sign, and paramagnetic rim lesions in multiple sclerosis: Emerging machine learning techniques and future avenues
Abstract
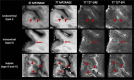
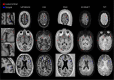
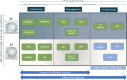
The current diagnostic criteria for multiple sclerosis (MS) lack specificity, and this may lead to misdiagnosis, which remains an issue in present-day clinical practice. In addition, conventional biomarkers only moderately correlate with MS disease progression. Recently, some MS lesional imaging biomarkers such as cortical lesions (CL), the central vein sign (CVS), and paramagnetic rim lesions (PRL), visible in specialized magnetic resonance imaging (MRI) sequences, have shown higher specificity in differential diagnosis. Moreover, studies have shown that CL and PRL are potential prognostic biomarkers, the former correlating with cognitive impairments and the latter with early disability progression. As machine learning-based methods have achieved extraordinary performance in the assessment of conventional imaging biomarkers, such as white matter lesion segmentation, several automated or semi-automated methods have been proposed as well for CL, PRL, and CVS. In the present review, we first introduce these MS biomarkers and their imaging methods. Subsequently, we describe the corresponding machine learning-based methods that were proposed to tackle these clinical questions, putting them into context with respect to the challenges they are facing, including non-standardized MRI protocols, limited datasets, and moderate inter-rater variability. We conclude by presenting the current limitations that prevent their broader deployment and suggesting future research directions.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The University Hospital Basel (USB), as the employer of C.G., has received the following fees which were used exclusively for research support: (i) advisory board and consultancy fees from Actelion, Genzyme-Sanofi, Novartis, GeNeuro and Roche; (ii) speaker feesfrom Genzyme-Sanofi, Novartis, GeNeuro and Roche; (iii) research support from Siemens, GeNeuro, Roche. M.A. has received consultancy fees from GSK and Sanofi-Genzyme. P.M. has received support from Biogen and Cliniques universitaires Saint-Luc Fonds de Recherche Clinique. D.S.R. has received research support from Abata, Sanofi-Genzyme, and Vertex. The other authors have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Antonelli M, Reinke A, Bakas S, et al. The Medical Segmentation Decathlon. ArXiv210605735 Cs Eess. Published online June 10, 2021. Accessed November 8, 2021. http://arxiv.org/abs/2106.05735.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

