A brain atlas of synapse protein lifetime across the mouse lifespan
- PMID: 36202095
- PMCID: PMC9789179
- DOI: 10.1016/j.neuron.2022.09.009
A brain atlas of synapse protein lifetime across the mouse lifespan
Abstract
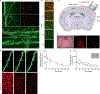
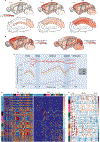
The lifetime of proteins in synapses is important for their signaling, maintenance, and remodeling, and for memory duration. We quantified the lifetime of endogenous PSD95, an abundant postsynaptic protein in excitatory synapses, at single-synapse resolution across the mouse brain and lifespan, generating the Protein Lifetime Synaptome Atlas. Excitatory synapses have a wide range of PSD95 lifetimes extending from hours to several months, with distinct spatial distributions in dendrites, neurons, and brain regions. Synapses with short protein lifetimes are enriched in young animals and in brain regions controlling innate behaviors, whereas synapses with long protein lifetimes accumulate during development, are enriched in the cortex and CA1 where memories are stored, and are preferentially preserved in old age. Synapse protein lifetime increases throughout the brain in a mouse model of autism and schizophrenia. Protein lifetime adds a further layer to synapse diversity and enriches prevailing concepts in brain development, aging, and disease.
Keywords: HaloTag; aging; autism; brain development; dendritic spine; postsynaptic density; protein turnover; proteostasis; pyramidal neuron; synapse proteome; synaptome.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



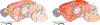
References
-
- Babiec WE, Jami SA, Guglietta R, Chen PB, and O’Dell TJ. (2017). Differential regulation of NMDA receptor-mediated transmission by SK channels underlies dorsal-ventral differences in dynamics of schaffer collateral synaptic function. J. Neurosci. 37, 1950–1964. 10.1523/JNEUROSCI.3196-16.2017 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

