Immune response after COVID-19 vaccination among patients with chronic kidney disease and kidney transplant
- PMID: 36202639
- PMCID: PMC9515331
- DOI: 10.1016/j.vaccine.2022.09.067
Immune response after COVID-19 vaccination among patients with chronic kidney disease and kidney transplant
Abstract
Background: Vaccination of patients with chronic kidney disease (CKD) and kidney transplants (KTs) may achieve a less robust immune response. Understanding such immune responses is crucial for guiding current and future vaccine dosing strategies.
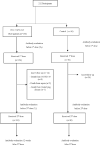
Methods: This prospective, observational study estimated the immunogenicity of humoral and cellular responses of two SARS-CoV-2 vaccines in different patient groups with CKD compared with controls. Secondary outcomes included adverse events after vaccination and the incidence of COVID-19 breakthrough infection, including illness severity.
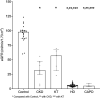
Results: In total, 212 patients received ChAdOx1 nCoV-19 (89.62 %) or inactivated vaccines (10.38 %).The antibody response against the S protein was analyzed at T0 (before the first injection), T1 (before the second injection), and T2 (12 weeks after the second injection). Seroconversion occurred in 92.31 % of controls at T2 and in 100 % of patients with CKD, 42.86 % undergoing KT, 80.18 % of hemodialysis (HD), and 0 % of patients undergoing continuous ambulatory peritoneal dialysis (CAPD) at T2 of the ChAdOx1 nCoV-19 vaccine. Neutralizing antibody levels by surrogate virus neutralization test were above the protective level at T2 in each group. The KT group exhibited the lowest neutralizing antibody and T cell response. Blood groups O and vaccine type were associated with good immunological responses. After the first dose, 14 individuals (6.6 out of the total population experienced COVID-19 breakthrough infection.
Conclusion: Immunity among patients with CKD and HD after vaccination was strong and comparable with that of healthy controls. Our study suggested that a single dose of the vaccine is not efficacious and delays may result in breakthrough infection. Some blood groups and types of vaccine can affect the immune response.
Keywords: Anti-spike RBD IgG; Chronic kidney disease; Coronavirus disease 2019 (COVID-19); Kidney transplant; Vaccine.
Copyright © 2022 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Thananda Trakarnvanich reports financial support was provided by Navamindradhiraj University. Thananda Trakarnvanich reports a relationship with Navamindradhiraj University that includes: funding grants and non-financial support. None to be declared.
Figures




References
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

