Selective Biocatalytic N-Methylation of Unsaturated Heterocycles
- PMID: 36202763
- PMCID: PMC9827881
- DOI: 10.1002/anie.202213056
Selective Biocatalytic N-Methylation of Unsaturated Heterocycles
Abstract
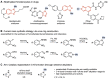
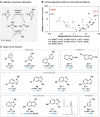
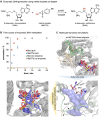
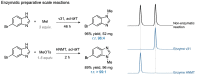
Methods for regioselective N-methylation and -alkylation of unsaturated heterocycles with "off the shelf" reagents are highly sought-after. This reaction could drastically simplify synthesis of privileged bioactive molecules. Here we report engineered and natural methyltransferases for challenging N-(m)ethylation of heterocycles, including benzimidazoles, benzotriazoles, imidazoles and indazoles. The reactions are performed through a cyclic enzyme cascade that consists of two methyltransferases using only iodoalkanes or methyl tosylate as simple reagents. This method enables the selective synthesis of important molecules that are otherwise difficult to access, proceeds with high regioselectivity (r.r. up to >99 %), yield (up to 99 %), on a preparative scale, and with nearly equimolar concentrations of simple starting materials.
Keywords: Alkylation; Biocatalysis; Heterocycles; Methyltransferase; SAM Recycling.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Taylor R. D., Maccoss M., Lawson A. D. G., J. Med. Chem. 2014, 57, 5845–5859. - PubMed
-
- Zhang S. G., Liang C. G., Zhang W. H., Molecules 2018, 23, 2783. - PubMed
-
- Faheem M., Rathaur A., Pandey A., Kumar Singh V., Tiwari A. K., ChemistrySelect 2020, 5, 3981–3994.
-
- Genung N. E., Wei L., Aspnes G. E., Org. Lett. 2014, 16, 3114–3117. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

