Effectiveness of an inactivated Covid-19 vaccine with homologous and heterologous boosters against Omicron in Brazil
- PMID: 36202800
- PMCID: PMC9537178
- DOI: 10.1038/s41467-022-33169-0
Effectiveness of an inactivated Covid-19 vaccine with homologous and heterologous boosters against Omicron in Brazil
Abstract
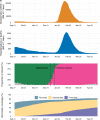
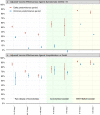
The effectiveness of inactivated vaccines (VE) against symptomatic and severe COVID-19 caused by omicron is unknown. We conducted a nationwide, test-negative, case-control study to estimate VE for homologous and heterologous (BNT162b2) booster doses in adults who received two doses of CoronaVac in Brazil in the Omicron context. Analyzing 1,386,544 matched-pairs, VE against symptomatic disease was 8.6% (95% CI, 5.6-11.5) and 56.8% (95% CI, 56.3-57.3) in the period 8-59 days after receiving a homologous and heterologous booster, respectively. During the same interval, VE against severe Covid-19 was 73.6% (95% CI, 63.9-80.7) and 86.0% (95% CI, 84.5-87.4) after receiving a homologous and heterologous booster, respectively. Waning against severe Covid-19 after 120 days was only observed after a homologous booster. Heterologous booster might be preferable to individuals with completed primary series inactivated vaccine.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

