A pathway for chitin oxidation in marine bacteria
- PMID: 36202810
- PMCID: PMC9537276
- DOI: 10.1038/s41467-022-33566-5
A pathway for chitin oxidation in marine bacteria
Abstract
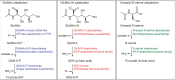
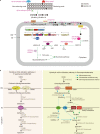
Oxidative degradation of chitin, initiated by lytic polysaccharide monooxygenases (LPMOs), contributes to microbial bioconversion of crystalline chitin, the second most abundant biopolymer in nature. However, our knowledge of oxidative chitin utilization pathways, beyond LPMOs, is very limited. Here, we describe a complete pathway for oxidative chitin degradation and its regulation in a marine bacterium, Pseudoalteromonas prydzensis. The pathway starts with LPMO-mediated extracellular breakdown of chitin into C1-oxidized chitooligosaccharides, which carry a terminal 2-(acetylamino)-2-deoxy-D-gluconic acid (GlcNAc1A). Transmembrane transport of oxidized chitooligosaccharides is followed by their hydrolysis in the periplasm, releasing GlcNAc1A, which is catabolized in the cytoplasm. This pathway differs from the known hydrolytic chitin utilization pathway in enzymes, transporters and regulators. In particular, GlcNAc1A is converted to 2-keto-3-deoxygluconate 6-phosphate, acetate and NH3 via a series of reactions resembling the degradation of D-amino acids rather than other monosaccharides. Furthermore, genomic and metagenomic analyses suggest that the chitin oxidative utilization pathway may be prevalent in marine Gammaproteobacteria.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Carbohydrate deacetylase, a key enzyme in oxidative chitin degradation, is evolutionarily linked to amino acid deacetylase.J Biol Chem. 2025 Apr;301(4):108420. doi: 10.1016/j.jbc.2025.108420. Epub 2025 Mar 18. J Biol Chem. 2025. PMID: 40113045 Free PMC article.
-
Chitin-Active Lytic Polysaccharide Monooxygenases Are Rare in Cellulomonas Species.Appl Environ Microbiol. 2022 Aug 9;88(15):e0096822. doi: 10.1128/aem.00968-22. Epub 2022 Jul 12. Appl Environ Microbiol. 2022. PMID: 35862679 Free PMC article.
-
The Fish Pathogen Aliivibrio salmonicida LFI1238 Can Degrade and Metabolize Chitin despite Gene Disruption in the Chitinolytic Pathway.Appl Environ Microbiol. 2021 Sep 10;87(19):e0052921. doi: 10.1128/AEM.00529-21. Epub 2021 Sep 10. Appl Environ Microbiol. 2021. PMID: 34319813 Free PMC article.
-
Chitin-Active Lytic Polysaccharide Monooxygenases.Adv Exp Med Biol. 2019;1142:115-129. doi: 10.1007/978-981-13-7318-3_6. Adv Exp Med Biol. 2019. PMID: 31102244 Review.
-
The interplay between lytic polysaccharide monooxygenases and glycoside hydrolases.Essays Biochem. 2023 Apr 18;67(3):551-559. doi: 10.1042/EBC20220156. Essays Biochem. 2023. PMID: 36876880 Review.
Cited by
-
The Ustilago maydis AA10 LPMO is active on fungal cell wall chitin.Appl Environ Microbiol. 2023 Oct 31;89(10):e0057323. doi: 10.1128/aem.00573-23. Epub 2023 Sep 13. Appl Environ Microbiol. 2023. PMID: 37702503 Free PMC article.
-
Chitinolytic and Fungicidal Potential of the Marine Bacterial Strains Habituating Pacific Ocean Regions.Microorganisms. 2023 Sep 8;11(9):2255. doi: 10.3390/microorganisms11092255. Microorganisms. 2023. PMID: 37764100 Free PMC article.
-
Molecular mechanism of the one-component regulator RccR on bacterial metabolism and virulence.Nucleic Acids Res. 2024 Apr 12;52(6):3433-3449. doi: 10.1093/nar/gkae171. Nucleic Acids Res. 2024. PMID: 38477394 Free PMC article.
-
Bacterial community function increases leaf growth in a pitcher plant experimental system.mSystems. 2024 Dec 17;9(12):e0129824. doi: 10.1128/msystems.01298-24. Epub 2024 Nov 25. mSystems. 2024. PMID: 39584840 Free PMC article.
-
Genomic Island-Encoded Diguanylate Cyclase from Vibrio alginolyticus Regulates Biofilm Formation and Motility in Pseudoalteromonas.Microorganisms. 2023 Nov 8;11(11):2725. doi: 10.3390/microorganisms11112725. Microorganisms. 2023. PMID: 38004737 Free PMC article.
References
-
- Muzzarelli, R. A. A. Chitin. (Pergamon Press, UK, 1977).
-
- Gooday GW. The ecology of chitin degradation. Adv. Microb. Ecol. 1990;11:387–430. doi: 10.1007/978-1-4684-7612-5_10. - DOI
-
- Tracey MA. Chitin. Rev. Pure Appl. Chem. 1957;7:1–13.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

