Cognitive benefits of using non-invasive compared to implantable neural feedback
- PMID: 36202893
- PMCID: PMC9537330
- DOI: 10.1038/s41598-022-21057-y
Cognitive benefits of using non-invasive compared to implantable neural feedback
Abstract
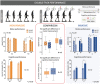
A non-optimal prosthesis integration into an amputee's body schema suggests some important functional and health consequences after lower limb amputation. These include low perception of a prosthesis as a part of the body, experiencing it as heavier than the natural limb, and cognitively exhausting use for users. Invasive approaches, exploiting the surgical implantation of electrodes in residual nerves, improved prosthesis integration by restoring natural and somatotopic sensory feedback in transfemoral amputees. A non-invasive alternative that avoids surgery would reduce costs and shorten certification time, significantly increasing the adoption of such systems. To explore this possibility, we compared results from a non-invasive, electro-cutaneous stimulation system to outcomes observed with the use of implants in above the knee amputees. This non-invasive solution was tested in transfemoral amputees through evaluation of their ability to perceive and recognize touch intensity and locations, or movements of a prosthesis, and its cognitive integration (through dual task performance and perceived prosthesis weight). While this managed to evoke the perception of different locations on the artificial foot, and closures of the leg, it was less performant than invasive solutions. Non-invasive stimulation induced similar improvements in dual motor and cognitive tasks compared to neural feedback. On the other hand, results demonstrate that remapped, evoked sensations are less informative and intuitive than the neural evoked somatotopic sensations. The device therefore fails to improve prosthesis embodiment together with its associated weight perception. This preliminary evaluation meaningfully highlights the drawbacks of non-invasive systems, but also demonstrates benefits when performing multiple tasks at once. Importantly, the improved dual task performance is consistent with invasive devices, taking steps towards the expedited development of a certified device for widespread use.
© 2022. The Author(s).
Conflict of interest statement
S.R. holds shares of “Sensars Neuroprosthetics”, a start-up company dealing with potential commercialization of neurocontrolled artificial limbs. The other authors do not have anything to disclose.
Figures




Similar articles
-
Lightening the Perceived Prosthesis Weight with Neural Embodiment Promoted by Sensory Feedback.Curr Biol. 2021 Mar 8;31(5):1065-1071.e4. doi: 10.1016/j.cub.2020.11.069. Epub 2021 Jan 7. Curr Biol. 2021. PMID: 33417885
-
Optimally-calibrated non-invasive feedback improves amputees' metabolic consumption, balance and walking confidence.J Neural Eng. 2022 Aug 25;19(4). doi: 10.1088/1741-2552/ac883b. J Neural Eng. 2022. PMID: 35944515
-
Discriminability of multiple cutaneous and proprioceptive hand percepts evoked by intraneural stimulation with Utah slanted electrode arrays in human amputees.J Neuroeng Rehabil. 2021 Jan 21;18(1):12. doi: 10.1186/s12984-021-00808-4. J Neuroeng Rehabil. 2021. PMID: 33478534 Free PMC article.
-
Sensory feedback for limb prostheses in amputees.Nat Mater. 2021 Jul;20(7):925-939. doi: 10.1038/s41563-021-00966-9. Epub 2021 Apr 15. Nat Mater. 2021. PMID: 33859381 Review.
-
A review of invasive and non-invasive sensory feedback in upper limb prostheses.Expert Rev Med Devices. 2017 Jun;14(6):439-447. doi: 10.1080/17434440.2017.1332989. Expert Rev Med Devices. 2017. PMID: 28532184 Review.
Cited by
-
Neuromorphic hardware for somatosensory neuroprostheses.Nat Commun. 2024 Jan 16;15(1):556. doi: 10.1038/s41467-024-44723-3. Nat Commun. 2024. PMID: 38228580 Free PMC article. Review.
-
Amputees but not healthy subjects optimally integrate non-spatially matched visuo-tactile stimuli.iScience. 2024 Dec 25;28(1):111685. doi: 10.1016/j.isci.2024.111685. eCollection 2025 Jan 17. iScience. 2024. PMID: 39886468 Free PMC article.
-
Wearable non-invasive neuroprosthesis for targeted sensory restoration in neuropathy.Nat Commun. 2024 Dec 30;15(1):10840. doi: 10.1038/s41467-024-55152-7. Nat Commun. 2024. PMID: 39738088 Free PMC article.
-
Closing the sensory feedback loop is necessary for effective neurorehabilitation.PLoS Biol. 2024 Oct 29;22(10):e3002866. doi: 10.1371/journal.pbio.3002866. eCollection 2024 Oct. PLoS Biol. 2024. PMID: 39471129 Free PMC article.
-
Symbiotic electroneural and musculoskeletal framework to encode proprioception via neurostimulation: ProprioStim.iScience. 2023 Feb 21;26(3):106248. doi: 10.1016/j.isci.2023.106248. eCollection 2023 Mar 17. iScience. 2023. PMID: 36923003 Free PMC article.
References
-
- Preatoni G, Valle G, Petrini FM, Raspopovic S. Report lightening the perceived prosthesis weight with neural embodiment promoted by sensory feedback. Curr. Biol. 2021;2021:1–7. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

