Murine foetal liver supports limited detectable expansion of life-long haematopoietic progenitors
- PMID: 36202972
- PMCID: PMC10026622
- DOI: 10.1038/s41556-022-00999-5
Murine foetal liver supports limited detectable expansion of life-long haematopoietic progenitors
Abstract
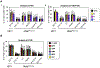
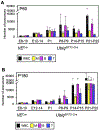
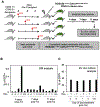
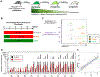
Current dogma asserts that the foetal liver (FL) is an expansion niche for recently specified haematopoietic stem cells (HSCs) during ontogeny. Indeed, between embryonic day of development (E)12.5 and E14.5, the number of transplantable HSCs in the murine FL expands from 50 to about 1,000. Here we used a non-invasive, multi-colour lineage tracing strategy to interrogate the embryonic expansion of murine haematopoietic progenitors destined to contribute to the adult HSC pool. Our data show that this pool of fated progenitors expands only two-fold during FL ontogeny. Although Histone2B-GFP retention in vivo experiments confirmed substantial proliferation of phenotypic FL-HSC between E12.5 and E14.5, paired-daughter cell assays revealed that many mid-gestation phenotypic FL-HSCs are biased to differentiate, rather than self-renew, relative to phenotypic neonatal and adult bone marrow HSCs. In total, these data support a model in which the FL-HSC pool fated to contribute to adult blood expands only modestly during ontogeny.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Declaration of Interests
The authors declare no competing interests.
Figures







References
-
- Kumaravelu P et al. Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): role of the aorta-gonad-mesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver. Development 129, 4891–4899 (2002). - PubMed
-
- Medvinsky A & Dzierzak E Definitive hematopoiesis is autonomously initiated by the AGM region. Cell 86, 897–906 (1996). - PubMed
-
- Muller AM, Medvinsky A, Strouboulis J, Grosveld F & Dzierzak E Development of hematopoietic stem cell activity in the mouse embryo. Immunity 1, 291–301 (1994). - PubMed
-
- North T et al. Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development 126, 2563–2575 (1999). - PubMed
-
- Yokomizo T et al. Requirement of Runx1/AML1/PEBP2alphaB for the generation of haematopoietic cells from endothelial cells. Genes Cells 6, 13–23 (2001). - PubMed
Methods-only References
-
- Benjamini Y, and Hochberg Y Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B 57, 289–300 (1995).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

