The complement system in pediatric acute kidney injury
- PMID: 36203104
- PMCID: PMC9540254
- DOI: 10.1007/s00467-022-05755-3
The complement system in pediatric acute kidney injury
Abstract
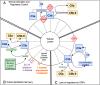
The complement cascade is an important part of the innate immune system. In addition to helping the body to eliminate pathogens, however, complement activation also contributes to the pathogenesis of a wide range of kidney diseases. Recent work has revealed that uncontrolled complement activation is the key driver of several rare kidney diseases in children, including atypical hemolytic uremic syndrome and C3 glomerulopathy. In addition, a growing body of literature has implicated complement in the pathogenesis of more common kidney diseases, including acute kidney injury (AKI). Complement-targeted therapeutics are in use for a variety of diseases, and an increasing number of therapeutic agents are under development. With the implication of complement in the pathogenesis of AKI, complement-targeted therapeutics could be trialed to prevent or treat this condition. In this review, we discuss the evidence that the complement system is activated in pediatric patients with AKI, and we review the role of complement proteins as biomarkers and therapeutic targets in patients with AKI.
Keywords: Acute kidney injury; Complement; Complement inhibitors.
© 2022. The Author(s), under exclusive licence to International Pediatric Nephrology Association.
Conflict of interest statement
Dr. Stenson and Dr. Kendrick declare they have no financial interests. Dr. Dixon is a consultant for Apellis and Alexion Pharmaceuticals, Inc. Dr. Thurman received royalties from Alexion Pharmaceuticals, Inc. and is a consultant for Q32 Bio, Inc., a company developing complement inhibitors. He also holds stock and will receive royalty income from Q32 Bio, Inc.
Figures


References
-
- Fitzgerald JC, Basu RK, Akcan-Arikan A, Izquierdo LM, Piñeres Olave BE, Hassinger AB, Szczepanska M, Deep A, Williams D, Sapru A, Roy JA, Nadkarni VM, Thomas N, SL JW, Furth S. Acute kidney injury in pediatric severe sepsis: an independent risk factor for death and new disability. Crit Care Med. 2016;44(12):2241–2250. doi: 10.1097/CCM.0000000000002007. - DOI - PMC - PubMed
-
- Alkandari O, Eddington KA, Hyder A, Gauvin F, Ducruet T, Gottesman R, Phan V, Zappitelli M. Acute kidney injury is an independent risk factor for pediatric intensive care unit mortality, longer length of stay and prolonged mechanical ventilation in critically ill children: a two-center retrospective cohort study. Critical Care. 2011;15(3):R146. doi: 10.1186/cc10269. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

