Histopathologically TMA-like distribution of multiple organ thromboses following the initial dose of the BNT162b2 mRNA vaccine (Comirnaty, Pfizer/BioNTech): an autopsy case report
- PMID: 36203145
- PMCID: PMC9540301
- DOI: 10.1186/s12959-022-00418-7
Histopathologically TMA-like distribution of multiple organ thromboses following the initial dose of the BNT162b2 mRNA vaccine (Comirnaty, Pfizer/BioNTech): an autopsy case report
Abstract
Background: Coronavirus disease 2019 (COVID-19) has spread worldwide. Vaccination is now recommended as one of the effective countermeasures to control the pandemic or prevent the worsening of symptoms. However, its adverse effects have been attracting attention. Here, we report an autopsy case of multiple thromboses after receiving the first dose of the BNT162b2 mRNA vaccine (Comirnaty, Pfizer/BioNTech) in an elderly woman.
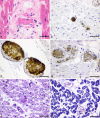
Case presentation: A 72-year-old woman with a history of diffuse large B-cell lymphoma in the stomach and hyperthyroidism received the first dose of the BNT162b2 mRNA vaccine and died 2 days later. The autopsy revealed multiple microthrombi in the heart, brain, liver, kidneys, and adrenal glands. The thrombi were CD61 and CD42b positive and were located in the blood vessels primarily in the pericardial aspect of the myocardium and subcapsular region of the adrenal glands; their diameters were approximately 5-40 μm. Macroscopically, a characteristic myocardial haemorrhage was observed, and the histopathology of the characteristic thrombus distribution, which differed from that of haemolytic uraemic syndrome and disseminated intravascular coagulation, suggested that the underlying pathophysiology may have been similar to that of thrombotic microangiopathy (TMA).
Conclusion: This is the first report on a post-mortem case of multiple thromboses after the BNT162b2 mRNA vaccine. The component thrombus and characteristic distribution of the thrombi were similar to those of TMA, which differs completely from haemolytic uraemic syndrome or disseminated intravascular coagulation, after vaccination. Although rare, it is important to consider that fatal adverse reactions may occur after vaccination and that it is vital to conduct careful follow-up.
Keywords: BNT162b2; Coronavirus disease 2019; SARS-CoV-2; Thrombosis; Thrombotic microangiopathy; Vaccination.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Status migrainosus: a potential adverse reaction to Comirnaty (BNT162b2, BioNtech/Pfizer) COVID-19 vaccine-a case report.Neurol Sci. 2022 Feb;43(2):767-770. doi: 10.1007/s10072-021-05741-x. Epub 2021 Nov 22. Neurol Sci. 2022. PMID: 34807361 Free PMC article.
-
Extensive Cerebral Venous Sinus Thrombosis (CVST) After the First Dose of Pfizer-BioNTech BNT162b2 mRNA COVID-19 Vaccine without Thrombotic Thrombocytopenia Syndrome (TTS) in a Healthy Woman.Am J Case Rep. 2022 Feb 9;23:e934744. doi: 10.12659/AJCR.934744. Am J Case Rep. 2022. PMID: 35136010 Free PMC article.
-
Patients With Autoimmune Thyroiditis Present Similar Immunological Response to COVID-19 BNT162b2 mRNA Vaccine With Healthy Subjects, While Vaccination May Affect Thyroid Function: A Clinical Study.Front Endocrinol (Lausanne). 2022 Feb 22;13:840668. doi: 10.3389/fendo.2022.840668. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35273575 Free PMC article.
-
COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines.Eur Rev Med Pharmacol Sci. 2021 Feb;25(3):1663-1669. doi: 10.26355/eurrev_202102_24877. Eur Rev Med Pharmacol Sci. 2021. PMID: 33629336 Review.
-
Rare Adverse Events Associated with BNT162b2 mRNA Vaccine (Pfizer-BioNTech): A Review of Large-Scale, Controlled Surveillance Studies.Vaccines (Basel). 2022 Jul 2;10(7):1067. doi: 10.3390/vaccines10071067. Vaccines (Basel). 2022. PMID: 35891231 Free PMC article. Review.
Cited by
-
Ignored dangers of the COVID-19 injections.Surg Neurol Int. 2023 Nov 3;14:385. doi: 10.25259/SNI_603_2023. eCollection 2023. Surg Neurol Int. 2023. PMID: 38053715 Free PMC article. No abstract available.
-
A case of biopsy-proven inflammatory dilated cardiomyopathy following heterologous mRNA-1273 third-dose immunization.ESC Heart Fail. 2024 Dec;11(6):4442-4449. doi: 10.1002/ehf2.14924. Epub 2024 Jul 1. ESC Heart Fail. 2024. PMID: 38946583 Free PMC article. No abstract available.
-
The Common Systemic and Local Adverse Effects of the Sinovac COVID-19 Vaccine: An Observational Study From Pakistan.Cureus. 2023 May 4;15(5):e38564. doi: 10.7759/cureus.38564. eCollection 2023 May. Cureus. 2023. PMID: 37284387 Free PMC article.
-
Side Effects of COVID-19 Vaccines Among Diabetic Subjects and Healthy Individuals.Cureus. 2023 Mar 10;15(3):e36005. doi: 10.7759/cureus.36005. eCollection 2023 Mar. Cureus. 2023. PMID: 37041898 Free PMC article.
-
End-Stage Kidney Disease Resulting from Atypical Hemolytic Uremic Syndrome after Receiving AstraZeneca SARS-CoV-2 Vaccine: A Case Report.Vaccines (Basel). 2023 Mar 16;11(3):679. doi: 10.3390/vaccines11030679. Vaccines (Basel). 2023. PMID: 36992263 Free PMC article.
References
-
- Ko JY, Danielson ML, Town M, Derado G, Greenlund KJ, Kirley PD, et al. Risk factors for coronavirus disease 2019 (COVID-19)-associated hospitalization: COVID-19-associated hospitalization surveillance network and behavioral risk factor surveillance system. Clin Infect Dis. 2021;72:e695–703. doi: 10.1093/cid/ciaa1419. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

