Risk of developing depression from endocrine treatment: A nationwide cohort study of women administered treatment for breast cancer in South Korea
- PMID: 36203445
- PMCID: PMC9530937
- DOI: 10.3389/fonc.2022.980197
Risk of developing depression from endocrine treatment: A nationwide cohort study of women administered treatment for breast cancer in South Korea
Abstract
Background: Although previous studies demonstrated no association between depression and tamoxifen in patients with breast cancer, there is still a limited amount of long-term follow-up data. This study aimed to evaluate the relationship between endocrine treatment and the risk of depression.
Methods: This nationwide population-based cohort study used data obtained over a 14-year period (January 2007 to December 2021) from the Korean National Health Insurance claims database. All female patients with breast cancer were included. We examined the incidence of depression in patients who underwent endocrine treatment, and those who did not undergo endocrine treatment constituted the control group.
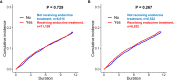
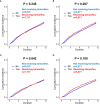
Results: The data from 11,109 patients who underwent endocrine treatment and 6,615 control patients between 2009 and 2010 were analyzed. After performing matching for comorbidities and age, both groups comprised 6,532 patients. The median follow-up were 119.71 months. Before and after matching was performed, the endocrine treatment was not a significant risk factor for developing depression (p=0.7295 and p=0.2668, respectively), nor was it a significant factor for an increased risk for suicide attempt (p=0.6381 and p=0.8366, respectively).
Conclusions: Using a real-world population-based cohort, this study demonstrated that there is no evidence that the endocrine treatment increases the risk of depression.
Keywords: aromatase inhibitor; breast cancer; depression; endocrine treatment; tamoxifen.
Copyright © 2022 Oh, Lee, Jeon, Kim, Seok, Park, Kim and Yoon.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Mitchell AJ, Chan M, Bhatti H, Halton M, Grassi L, Johansen C, et al. . Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: A meta-analysis of 94 interview-based studies. Lancet Oncol (2011) 12(2):160–74. doi: 10.1016/S1470-2045(11)70002-X - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

