Time-dependent contraction of the SARS-CoV-2-specific T-cell responses in convalescent individuals
- PMID: 36203479
- PMCID: PMC9170273
- DOI: 10.1016/j.jacig.2022.05.002
Time-dependent contraction of the SARS-CoV-2-specific T-cell responses in convalescent individuals
Abstract
Background: Adaptive immunity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection is decisive for disease control. Delayed activation of T cells is associated with a worse outcome in coronavirus disease 2019 (COVID-19). Although convalescent individuals exhibit solid T-cell immunity, to date, long-term immunity to SARS-CoV-2 is still under investigation.
Objectives: We aimed to characterize the specific T-cell response on the basis of the in vitro recall of IFN-γ-producing cells to in silico-predicted peptides in samples from SARS-CoV-2 convalescent individuals.
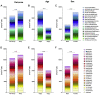
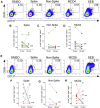
Methods: The sequence of the SARS-CoV-2 genome was screened, leading to the identification of specific and promiscuous peptides predicted to be recognized by CD4+ and CD8+ T cells. Next, we performed an in vitro recall of specific T cells from PBMC samples from the participants. The results were analyzed according to clinical features of the cohort and HLA diversity.
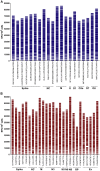
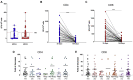
Results: Our results indicated heterogeneous T-cell responsiveness among the participants. Compared with patients who exhibited mild symptoms, hospitalized patients had a significantly higher magnitude of response. In addition, male and older patients showed a lower number of IFN-γ-producing cells. Analysis of samples collected after 180 days revealed a reduction in the number of specific circulating IFN-γ-producing T cells, suggesting decreased immunity against viral peptides.
Conclusion: Our data are evidence that in silico-predicted peptides are highly recognized by T cells from convalescent individuals, suggesting a possible application for vaccine design. However, the number of specific T cells decreases 180 days after infection, which might be associated with reduced protection against reinfection over time.
Keywords: AIM, Activation-induced marker; COVID-19; COVID-19, Coronavirus disease 2019; DMSO, Dimethyl sulfoxide; ELISPOT, Enzyme-linked immunospot; OR, Odds ratio; SARS-CoV-2; SARS-CoV-2, Severe acute respiratory syndrome coronavirus-2; SFU, Spot-forming unit; T lymphocyte; VOC, Variant of concern; adaptive immunity.
© 2022 The Authors.
Figures
References
-
- Gandhi R.T., Lynch J.B., Del Rio C. Mild or moderate Covid-19. N Engl J Med. 2020;383:1757–1766. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

