Budesonide-Loaded Hyaluronic Acid Nanoparticles for Targeted Delivery to the Inflamed Intestinal Mucosa in a Rodent Model of Colitis
- PMID: 36203483
- PMCID: PMC9532096
- DOI: 10.1155/2022/7776092
Budesonide-Loaded Hyaluronic Acid Nanoparticles for Targeted Delivery to the Inflamed Intestinal Mucosa in a Rodent Model of Colitis
Abstract
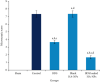
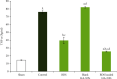
The aim of the present study was to investigate the therapeutic potential of budesonide- (BDS-) loaded hyaluronic acid nanoparticles (HANPs) for treatment of inflammatory bowel disease (IBD) using an acute model of colitis in rats. The therapeutic efficacy of BDS-loaded HANPs in comparison with an aqueous suspension of the drug with the same dose (30 μg/kg) was investigated 48 h following induction of colitis by intrarectal administration of acetic acid 4% in rats. Microscopic and histopathologic examinations were conducted in inflamed colonic tissue. Tissue concentration of tumor necrosis factor (TNF)-α was assessed by ELISA assay kit, while the activity of myeloperoxidase (MPO) was measured spectrophotometrically. Results from in vivo evaluations demonstrated that administrations of BDS-HANPs ameliorated the general endoscopic appearance, quite close to the healthy animals with no signs of inflammation and reduced the cellular infiltration, as well as the TNF-α level, and the MPO activity. It was found that delivery by BDS-loaded HANPSs alleviated the induced colitis significantly better than the same dose of the free drug. These data further suggest the potential of HANPs as a targeted drug delivery system to the inflamed colon mucosa.
Copyright © 2022 Seyed Yaser Vafaei et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





Similar articles
-
Self assembled hyaluronic acid nanoparticles as a potential carrier for targeting the inflamed intestinal mucosa.Carbohydr Polym. 2016 Jun 25;144:371-81. doi: 10.1016/j.carbpol.2016.01.026. Epub 2016 Jan 25. Carbohydr Polym. 2016. PMID: 27083829
-
Budesonide-loaded nanostructured lipid carriers reduce inflammation in murine DSS-induced colitis.Int J Pharm. 2013 Oct 1;454(2):775-83. doi: 10.1016/j.ijpharm.2013.05.017. Epub 2013 May 18. Int J Pharm. 2013. PMID: 23694806
-
Colon-specific delivery of budesonide from microencapsulated cellulosic cores: evaluation of the efficacy against colonic inflammation in rats.J Pharm Pharmacol. 2001 Sep;53(9):1207-15. doi: 10.1211/0022357011776658. J Pharm Pharmacol. 2001. PMID: 11578103
-
Budesonide Loaded PLGA Nanoparticles for Targeting the Inflamed Intestinal Mucosa--Pharmaceutical Characterization and Fluorescence Imaging.Pharm Res. 2016 May;33(5):1085-92. doi: 10.1007/s11095-015-1852-6. Epub 2015 Dec 30. Pharm Res. 2016. PMID: 26718953
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
Cited by
-
Targeting the Gut: A Systematic Review of Specific Drug Nanocarriers.Pharmaceutics. 2024 Mar 21;16(3):431. doi: 10.3390/pharmaceutics16030431. Pharmaceutics. 2024. PMID: 38543324 Free PMC article. Review.
-
Targeted delivery of budesonide in acetic acid induced colitis: impact on miR-21 and E-cadherin expression.Drug Deliv Transl Res. 2023 Nov;13(11):2930-2947. doi: 10.1007/s13346-023-01363-2. Epub 2023 May 15. Drug Deliv Transl Res. 2023. PMID: 37184747 Free PMC article.
-
Recognizing the biological barriers and pathophysiological characteristics of the gastrointestinal tract for the design and application of nanotherapeutics.Drug Deliv. 2024 Dec;31(1):2415580. doi: 10.1080/10717544.2024.2415580. Epub 2024 Oct 15. Drug Deliv. 2024. PMID: 39404464 Free PMC article. Review.
-
The Modulation of Cell Plasticity by Budesonide: Beyond the Metabolic and Anti-Inflammatory Actions of Glucocorticoids.Pharmaceutics. 2025 Apr 11;17(4):504. doi: 10.3390/pharmaceutics17040504. Pharmaceutics. 2025. PMID: 40284499 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

