A Pre-clinical Standard Operating Procedure for Evaluating Orthobiologics in an In Vivo Rat Spinal Fusion Model
- PMID: 36203492
- PMCID: PMC9534599
- DOI: 10.26502/josm.511500060
A Pre-clinical Standard Operating Procedure for Evaluating Orthobiologics in an In Vivo Rat Spinal Fusion Model
Abstract
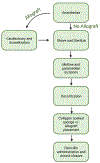
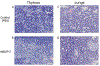
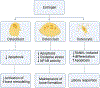
The rat animal model is a cost effective and reliable model used in spinal pre-clinical research. Complications from various surgical procedures in humans often arise that were based on these pre-clinical animal models. Therefore safe and efficacious pre-clinical animal models are needed to establish continuity into clinical trials. A Standard Operating Procedure (SOP) is a validated method that allows researchers to safely and carefully replicate previously successful surgical techniques. Thus, the aim of this study is to describe in detail the procedures involved in a common rat bilateral posterolateral intertransverse spinal fusion SOP used to test the efficacy and safety different orthobiologics using a collagen-soaked sponge as an orthobiologic carrier. Only two orthobiologics are currently FDA approved for spinal fusion surgery which include recombinant bone morphogenetic protein 2 (rhBMP-2), and I-FACTOR. While there are many additional orthobiologics currently being tested, one way to show their safety profile and gain FDA approval, is to use well established pre-clinical animal models. A preoperative, intraoperative, and postoperative surgical setup including specific anesthesia and euthanasia protocols are outlined. Furthermore, we describe different postoperative methods used to validate the spinal fusion SOP, which include μCT analysis, histopathology, biomechanical testing, and blood analysis. This SOP can help increase validity, transparency, efficacy, and reproducibly in future rat spinal fusion surgery procedures.
Keywords: Orthobiologics; Posterolateral intertransverse fusion model; Protocols; Rat model; Spinal fusion; Standard Operating Procedure (SOP); rhBMP-2.
Conflict of interest statement
Conflicts of Interest The authors have no conflicts of interest to declare.
Figures

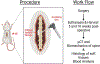



References
-
- Schnake KJ, Rappert D, Storzer B, et al. Lumbar fusion-Indications and techniques. Orthopade 48 (2019): 50–58. - PubMed
-
- Weiss AJ, Elixhauser A. Trends in Operating Room Procedures in U.S. Hospitals, 2001–2011: Statistical Brief #171, in Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality (US): Rockville (MD) (2006). - PubMed
-
- Dasta JF, McLaughlin TP, Mody SH, et al. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med 33 (2005): 1266–12 71. - PubMed
-
- White MS, Burns C, Conlon HA. The Impact of an Aging Population in the Workplace. Workplace Health Saf 66 (2018): 493–498. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
