Saliva is suitable for SARS-CoV-2 antibodies detection after vaccination: A rapid systematic review
- PMID: 36203571
- PMCID: PMC9530471
- DOI: 10.3389/fimmu.2022.1006040
Saliva is suitable for SARS-CoV-2 antibodies detection after vaccination: A rapid systematic review
Abstract
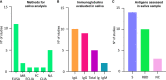
Since the introduction of efficient vaccines anti-SARS-CoV-2, antibody quantification becomes increasingly useful for immunological monitoring and COVID-19 control. In several situations, saliva samples may be an alternative to the serological test. Thus, this rapid systematic review aimed to evaluate if saliva is suitable for SARS-CoV-2 detection after vaccination. For this purpose, search strategies were applied at EMBASE, PubMed, and Web of Science. Studies were selected by two reviewers in a two-phase process. After selection, 15 studies were eligible and included in data synthesis. In total, salivary samples of approximately 1,080 vaccinated and/or convalescent individuals were analyzed. The applied vaccines were mostly mRNA-based (BioNTech 162b2 mRNA/Pfizer and Spikevax mRNA-1273/Moderna), but recombinant viral-vectored vaccines (Ad26. COV2. S Janssen - Johnson & Johnson and Vaxzevria/Oxford AstraZeneca) were also included. Different techniques were applied for saliva evaluation, such as ELISA assay, Multiplex immunoassay, flow cytometry, neutralizing and electrochemical assays. Although antibody titers are lower in saliva than in serum, the results showed that saliva is suitable for antibody detection. The mean of reported correlations for titers in saliva and serum/plasma were moderate for IgG (0.55, 95% CI 0.38-9.73), and weak for IgA (0.28, 95% CI 0.12-0.44). Additionally, six out of nine studies reported numerical titers for immunoglobulins detection, from which the level in saliva reached their reference value in four (66%). IgG but not IgA are frequently presented in saliva from vaccinated anti-COVID-19. Four studies reported lower IgA salivary titers in vaccinated compared to previously infected individuals, otherwise, two reported higher titers of IgA in vaccinated. Concerning IgG, two studies reported high antibody titers in the saliva of vaccinated individuals compared to those previously infected and one presented similar results for vaccinated and infected. The detection of antibodies anti-SARS-CoV-2 in the saliva is available, which suggests this type of sample is a suitable alternative for monitoring the population. Thus, the results also pointed out the possible lack of mucosal immunity induction after anti-SARS-CoV-2 vaccination. It highlights the importance of new vaccination strategies also focused on mucosal alternatives directly on primary routes of SARS-CoV-2 entrance.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022336968, identifier CRD42022336968.
Keywords: COVID-19 vaccines; IgA; IgG; SARS-CoV-2; antibodies; saliva.
Copyright © 2022 Guerra, Castro, Amorim dos Santos, Acevedo and Chardin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Detection of anti-SARS-CoV-2 salivary antibodies in vaccinated adults.Front Immunol. 2023 Nov 7;14:1296603. doi: 10.3389/fimmu.2023.1296603. eCollection 2023. Front Immunol. 2023. PMID: 38022522 Free PMC article.
-
Comparison of IgA, IgG, and Neutralizing Antibody Responses Following Immunization With Moderna, BioNTech, AstraZeneca, Sputnik-V, Johnson and Johnson, and Sinopharm's COVID-19 Vaccines.Front Immunol. 2022 Jun 21;13:917905. doi: 10.3389/fimmu.2022.917905. eCollection 2022. Front Immunol. 2022. PMID: 35799790 Free PMC article.
-
Serum and Salivary IgG and IgA Response After COVID-19 Messenger RNA Vaccination.JAMA Netw Open. 2024 Apr 1;7(4):e248051. doi: 10.1001/jamanetworkopen.2024.8051. JAMA Netw Open. 2024. PMID: 38652471 Free PMC article.
-
Clinical Assessment of SARS-CoV-2 Antibodies in Oral Fluids Following Infection and Vaccination.Clin Chem. 2024 Apr 3;70(4):589-596. doi: 10.1093/clinchem/hvad169. Clin Chem. 2024. PMID: 38039096 Free PMC article. Review.
-
Association of SARS-CoV-2 Vaccination or Infection With Bell Palsy: A Systematic Review and Meta-analysis.JAMA Otolaryngol Head Neck Surg. 2023 Jun 1;149(6):493-504. doi: 10.1001/jamaoto.2023.0160. JAMA Otolaryngol Head Neck Surg. 2023. PMID: 37103913 Free PMC article.
Cited by
-
Characteristics of Vaccine- and Infection-Induced Systemic IgA Anti-SARS-CoV-2 Spike Responses.Vaccines (Basel). 2023 Sep 7;11(9):1462. doi: 10.3390/vaccines11091462. Vaccines (Basel). 2023. PMID: 37766138 Free PMC article.
-
Mapping IgA Epitope and Cross-Reactivity between Severe Acute Respiratory Syndrome-Associated Coronavirus 2 and DENV.Vaccines (Basel). 2023 Nov 24;11(12):1749. doi: 10.3390/vaccines11121749. Vaccines (Basel). 2023. PMID: 38140154 Free PMC article.
-
Long-term systemic and mucosal SARS-CoV-2 IgA response and its association with persistent smell and taste disorders.Front Immunol. 2023 Mar 8;14:1140714. doi: 10.3389/fimmu.2023.1140714. eCollection 2023. Front Immunol. 2023. PMID: 36969158 Free PMC article.
-
Detection of anti-SARS-CoV-2 salivary antibodies in vaccinated adults.Front Immunol. 2023 Nov 7;14:1296603. doi: 10.3389/fimmu.2023.1296603. eCollection 2023. Front Immunol. 2023. PMID: 38022522 Free PMC article.
-
Development of an Oral IgA Response against SARS-CoV-2 Following Immunization with Different COVID-19 Vaccines.Viruses. 2023 Nov 25;15(12):2319. doi: 10.3390/v15122319. Viruses. 2023. PMID: 38140560 Free PMC article.
References
-
- WHO . Annexes to the recommendations for use of vaccines against COVID-19 (Accessed 15 Jun 2022).
-
- Ritchie H, Mathieu E, Rodés-Guirao L, Appel C, Giattino C, Ortiz-Ospina E, et al. “Coronavirus pandemic (COVID-19).” Our world in data. OurWorldInData.org; (2020). Available at: https://ourworldindata.org/coronavirus.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

