Rethinking detection of pre-existing and intervening Plasmodium infections in malaria clinical trials
- PMID: 36203582
- PMCID: PMC9531235
- DOI: 10.3389/fimmu.2022.1003452
Rethinking detection of pre-existing and intervening Plasmodium infections in malaria clinical trials
Abstract
Pre-existing and intervening low-density Plasmodium infections complicate the conduct of malaria clinical trials. These infections confound infection detection endpoints, and their immunological effects may detract from intended vaccine-induced immune responses. Historically, these infections were often unrecognized since infrequent and often analytically insensitive parasitological testing was performed before and during trials. Molecular diagnostics now permits their detection, but investigators must weigh the cost, complexity, and personnel demands on the study and the laboratory when scheduling such tests. This paper discusses the effect of pre-existing and intervening, low-density Plasmodium infections on malaria vaccine trial endpoints and the current methods employed for their infection detection. We review detection techniques, that until recently, provided a dearth of cost-effective strategies for detecting low density infections. A recently deployed, field-tested, simple, and cost-effective molecular diagnostic strategy for detecting pre-existing and intervening Plasmodium infections from dried blood spots (DBS) in malaria-endemic settings is discussed to inform new clinical trial designs. Strategies that combine sensitive molecular diagnostic techniques with convenient DBS collections and cost-effective pooling strategies may enable more thorough and informative infection monitoring in upcoming malaria clinical trials and epidemiological studies.
Keywords: 18S rRNA; Plasmodium falciparum; at-home DBS; clinical trial; intervening infection; pre-existing infection.
Copyright © 2022 Owalla, Hergott, Seilie, Staubus, Chavtur, Chang, Kublin, Egwang and Murphy.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
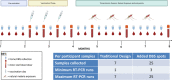
References
-
- RTS,S Clinical Trials Partnership . Efficacy and safety of the RTS, S/AS01 malaria vaccine during 18 months after vaccination: A phase 3 randomized, controlled trial in children and young infants at 11 African sites. PloS Med (2014) 11(7):e1001685. doi: 10.1371/journal.pmed.1001685 - DOI - PMC - PubMed
-
- Datoo MS, Natama MH, Some A, Traore O, Rouamba T, Bellamy D, et al. . Efficacy of a low-dose candidate malaria vaccine, R21 in adjuvant Matrix-M, with seasonal administration to children in Burkina Faso: A randomised controlled trial. Lancet (2021) 397(10287):1809–18. doi: 10.1016/S0140-6736(21)00943-0 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

