Body mass index, as a novel predictor of hepatocellular carcinoma patients treated with Anti-PD-1 immunotherapy
- PMID: 36203764
- PMCID: PMC9530364
- DOI: 10.3389/fmed.2022.981001
Body mass index, as a novel predictor of hepatocellular carcinoma patients treated with Anti-PD-1 immunotherapy
Abstract
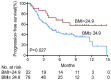
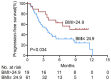
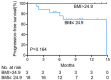
Immunocheckpoint inhibitors have shown significant efficacy in the treatment of hepatocellular carcinoma (HCC), but there are individual differences. The aim of this study was to explore body mass index (BMI) as a predictor of anti-PD-1 efficacy in patients with HCC. We retrospectively analyzed 101 HCC patients who treated with anti-PD-1 at Sun Yat-sen University Cancer Center from July 2018 to November 2019 and divided them into overweight (BMI > 24.9) and non-overweight (BMI ≤ 24.9) groups based on baseline BMI levels. BMI > 24.9 accounted for 22 cases (21.8%) and BMI ≤ 24.9 accounted for 79 cases (78.2%) in the study cohort. Overweight patients had higher disease control rates than non-overweight patients (P = 0.019, respectively). The mean progression-free survival (PFS) in overweight patients (10.23 months) was significantly longer than that of non-overweight patients (6.85 months; P = 0.027). Among patients with immune-related adverse events (irAEs), the mean PFS was also significantly longer in overweight patients (7.72 months) than in non-overweight patients (5.31 months, P = 0.034). Multivariate analysis showed that BMI was an independent prognostic factor for PFS in HCC patients treated with anti-PD-1 (hazard ratio: 0.47, P = 0.044). Thus, higher BMI predicts a better prognosis among HCC patients treated with anti-PD-1. In clinical practice, patients' BMI can provide a useful tool for predicting the efficacy of anti-PD-1 therapy.
Keywords: anti-PD-1 antibody; body mass index; hepatocellular carcinoma; immune-related adverse events; progression-free survival.
Copyright © 2022 Chen, Lu, Qu, A, Deng, Cai, Chen, Zheng and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Body mass index is not associated with survival outcomes and immune-related adverse events in patients with Hodgkin lymphoma treated with the immune checkpoint inhibitor nivolumab.J Transl Med. 2021 Dec 1;19(1):489. doi: 10.1186/s12967-021-03134-4. J Transl Med. 2021. PMID: 34852840 Free PMC article.
-
Immune-related adverse events predict responses to PD-1 blockade immunotherapy in hepatocellular carcinoma.Int J Cancer. 2021 Apr 22. doi: 10.1002/ijc.33609. Online ahead of print. Int J Cancer. 2021. PMID: 33890283
-
Another side of the association between body mass index (BMI) and clinical outcomes of cancer patients receiving programmed cell death protein-1 (PD-1)/ Programmed cell death-ligand 1 (PD-L1) checkpoint inhibitors: A multicentre analysis of immune-related adverse events.Eur J Cancer. 2020 Mar;128:17-26. doi: 10.1016/j.ejca.2019.12.031. Epub 2020 Mar 5. Eur J Cancer. 2020. PMID: 32109847
-
Clinical benefits of PD-1/PD-L1 inhibitors in advanced hepatocellular carcinoma: a systematic review and meta-analysis.Hepatol Int. 2020 Sep;14(5):765-775. doi: 10.1007/s12072-020-10064-8. Epub 2020 Jun 22. Hepatol Int. 2020. PMID: 32572818
-
Is Higher BMI Associated with Worse Overall Mortality in Hepatocellular Carcinoma Patients? An Evidence Based Case Report.Acta Med Indones. 2019 Oct;51(4):356-363. Acta Med Indones. 2019. PMID: 32041922 Review.
Cited by
-
Body composition predicts prognosis of hepatocellular carcinoma patients undergoing immune checkpoint inhibitors.J Cancer Res Clin Oncol. 2023 Oct;149(13):11607-11617. doi: 10.1007/s00432-023-05051-z. Epub 2023 Jul 4. J Cancer Res Clin Oncol. 2023. PMID: 37400572 Free PMC article.
-
Obesity contributes to hepatocellular carcinoma development via immunosuppressive microenvironment remodeling.Front Immunol. 2023 May 17;14:1166440. doi: 10.3389/fimmu.2023.1166440. eCollection 2023. Front Immunol. 2023. PMID: 37266440 Free PMC article. Review.
-
Obesity, the Adipose Organ and Cancer in Humans: Association or Causation?Biomedicines. 2023 Apr 28;11(5):1319. doi: 10.3390/biomedicines11051319. Biomedicines. 2023. PMID: 37238992 Free PMC article. Review.
-
Prognostic value of inflammatory and nutritional indexes among patients with unresectable advanced gastric cancer receiving immune checkpoint inhibitors combined with chemotherapy-a retrospective study.PeerJ. 2024 Dec 17;12:e18659. doi: 10.7717/peerj.18659. eCollection 2024. PeerJ. 2024. PMID: 39713151 Free PMC article.
References
-
- El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. . Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. (2017) 389:2492–502. 10.1016/S0140-6736(17)31046-2 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

