Crosstalk between the Intestinal Virome and Other Components of the Microbiota, and Its Effect on Intestinal Mucosal Response and Diseases
- PMID: 36203793
- PMCID: PMC9532165
- DOI: 10.1155/2022/7883945
Crosstalk between the Intestinal Virome and Other Components of the Microbiota, and Its Effect on Intestinal Mucosal Response and Diseases
Abstract
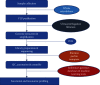
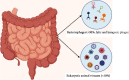
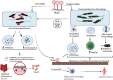
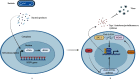
In recent years, there has been ample evidence illustrating the effect of microbiota on gut immunity, homeostasis, and disease. Most of these studies have engaged more efforts in understanding the role of the bacteriome in gut mucosal immunity and disease. However, studies on the virome and its influence on gut mucosal immunity and pathology are still at infancy owing to limited metagenomic tools. Nonetheless, the existing studies on the virome have largely been focused on the bacteriophages as these represent the main component of the virome with little information on endogenous retroviruses (ERVs) and eukaryotic viruses. In this review, we describe the gut virome, and its role in gut mucosal response and disease progression. We also explore the crosstalk between the virome and other microorganisms in the gut mucosa and elaborate on how these interactions shape the gut mucosal immunity going from bacteriophages through ERVs to eukaryotic viruses. Finally, we elucidate the potential contribution of this crosstalk in the pathogenesis of inflammatory bowel diseases and colon cancer.
Copyright © 2022 Njinju Asaba Clinton et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

