The AMPK pathway in fatty liver disease
- PMID: 36203933
- PMCID: PMC9531345
- DOI: 10.3389/fphys.2022.970292
The AMPK pathway in fatty liver disease
Abstract
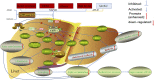
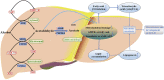
Lipid metabolism disorders are the primary causes for the occurrence and progression of various liver diseases, including non-alcoholic fatty liver disease (NAFLD) and alcoholic fatty liver disease (AFLD) caused by a high-fat diet and ethanol. AMPK signaling pathway plays an important role in ameliorating lipid metabolism disorders. Progressive research has clarified that AMPK signal axes are involved in the prevention and reduction of liver injury. Upregulation of AMK can alleviate FLD in mice induced by alcohol or insulin resistance, type 2 diabetes, and obesity, and most natural AMPK agonists can regulate lipid metabolism, inflammation, and oxidative stress in hepatocytes, consequently regulating FLD in mice. In NAFLD and AFLD, increasing the activity of AMPK can inhibit the synthesis of fatty acids and cholesterol by down-regulating the expression of adipogenesis gene (FAS, SREBP-1c, ACC and HMGCR); Simultaneously, by increasing the expression of fatty acid oxidation and lipid decomposition genes (CPT1, PGC1, and HSL, ATGL) involved in fatty acid oxidation and lipid decomposition, the body's natural lipid balance can be maintained. At present, some AMPK activators are thought to be beneficial during therapeutic treatment. Therefore, activation of AMPK signaling pathway is a potential therapeutic target for disorders of the liver. We summarized the most recent research on the role of the AMPK pathway in FLD in this review. Simultaneously, we performed a detailed description of each signaling axis of the AMPK pathway, as well as a discussion of its mechanism of action and therapeutic significance.
Keywords: AMPK signaling pathway; alcoholic fatty liver; lipid accumulation; lipid metabolism; non-alcoholic fatty liver.
Copyright © 2022 Fang, Pan, Qu, Lei, Han, Zhang and Han.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

