Metformin modulates the gut microbiome in broiler breeder hens
- PMID: 36203937
- PMCID: PMC9531308
- DOI: 10.3389/fphys.2022.1000144
Metformin modulates the gut microbiome in broiler breeder hens
Abstract
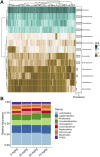
Broiler breeder hens, the parent stock of commercial broiler chickens, are genetically selected for rapid growth. Due to a longer production period and the focus of genetic selection on superior carcass traits in their progeny, these hens have the propensity to gain excess adipose tissue and exhibit severe ovarian dysfunction, a phenotype that is similar to human polycystic ovary syndrome (PCOS). Metformin is an antihyperglycemic drug approved for type 2 diabetes that is prescribed off-label for PCOS with benefits on metabolic and reproductive health. An additional effect of metformin treatments in humans is modulation of gut microbiome composition, hypothesized to benefit glucose sensitivity and systemic inflammation. The effects of dietary metformin supplementation in broiler breeder hens have not been investigated, thus we hypothesized that dietary metformin supplementation would alter the gut microbiome of broiler breeder hens. Broiler breeder hens were supplemented with metformin at four different levels (0, 25, 50, and 75 mg/kg body weight) from 25 to 65 weeks of age, and a subset of hens (n = 8-10 per treatment group) was randomly selected to undergo longitudinal microbiome profiling with 16S rRNA sequencing. Metformin impacted the microbial community composition in 75 mg/kg metformin compared to controls (adjusted PERMANOVA p = 0.0006) and an additional dose-dependent difference was observed between 25 mg/kg and 75 mg/kg (adjusted PERMANOVA p = 0.001) and between 50 mg/kg and 75 mg/kg (adjusted PERMANOVA p = 0.001) but not between 25 mg/kg and 50 mg/kg (adjusted PERMANOVA p = 0.863). There were few differences in the microbiome attributed to hen age, and metformin supplementation did not alter alpha diversity. Bacteria that were identified as differentially relatively abundant between 75 mg/kg metformin treatment and the control, and between metformin doses, included Ruminococcus and members of the Clostridia family that have been previously identified in human trials of PCOS. These results demonstrate that metformin impacts the microbiome of broiler breeder hens in a dose-dependent manner and several findings were consistent with PCOS in humans and with metformin treatment in type 2 diabetes. Metformin supplementation is a potentially promising option to improve gut health and reproductive efficiency in broiler breeder hens.
Keywords: broiler breeder hens; gut microbiome; metformin; polycystic ovary syndrome (PCOS); poultry.
Copyright © 2022 Van Syoc, Weaver, Rogers, Silverman, Ramachandran and Ganda.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Andrews S. (2010). FastQC: A quality control tool for high throughput sequence data. Available at: http:/www.bioinformatics.babraham.ac.uk/projects/fastqc/ .
-
- Barnett D. M., Arts I. C. W., Penders J. (2021). microViz: an R package for microbiome data visualization and statistics. J. Open Source Softw. 6, 3201. 10.21105/joss.03201 - DOI
LinkOut - more resources
Full Text Sources

