Piezoresistive MXene/Silk fibroin nanocomposite hydrogel for accelerating bone regeneration by Re-establishing electrical microenvironment
- PMID: 36203961
- PMCID: PMC9513113
- DOI: 10.1016/j.bioactmat.2022.08.025
Piezoresistive MXene/Silk fibroin nanocomposite hydrogel for accelerating bone regeneration by Re-establishing electrical microenvironment
Abstract
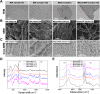
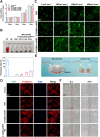
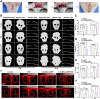
The electrical microenvironment plays an important role in bone repair. However, the underlying mechanism by which electrical stimulation (ES) promotes bone regeneration remains unclear, limiting the design of bone microenvironment-specific electroactive materials. Herein, by simple co-incubation in aqueous suspensions at physiological temperatures, biocompatible regenerated silk fibroin (RSF) is found to assemble into nanofibrils with a β-sheet structure on MXene nanosheets, which has been reported to inhibit the restacking and oxidation of MXene. An electroactive hydrogel based on RSF and bioencapsulated MXene is thus prepared to promote efficient bone regeneration. This MXene/RSF hydrogel also acts as a piezoresistive pressure transducer, which can potentially be utilized to monitor the electrophysiological microenvironment. RNA sequencing is performed to explore the underlying mechanisms, which can activate Ca2+/CALM signaling in favor of the direct osteogenesis process. ES is found to facilitate indirect osteogenesis by promoting the polarization of M2 macrophages, as well as stimulating the neogenesis and migration of endotheliocytes. Consistent improvements in bone regeneration and angiogenesis are observed with MXene/RSF hydrogels under ES in vivo. Collectively, the MXene/RSF hydrogel provides a distinctive and promising strategy for promoting direct osteogenesis, regulating immune microenvironment and neovascularization under ES, leading to re-establish electrical microenvironment for bone regeneration.
Keywords: Bone regeneration; Electrical microenvironment; Electrical stimulation; MXene; Regenerated silk fibroin.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures








References
-
- Rajabi A.H., Jaffe M., Arinzeh T.L. Piezoelectric materials for tissue regeneration: a review. Acta Biomater. 2015;24:12–23. - PubMed
-
- Thrivikraman G., Lee P.S., Hess R., Haenchen V., Basu B., Scharnweber D. Interplay of substrate conductivity, cellular microenvironment, and pulsatile electrical stimulation toward osteogenesis of human mesenchymal stem cells in vitro. ACS Appl. Mater. Interfaces. 2015;7(41):23015–23028. - PubMed
-
- Mollon B., da Silva V., Busse J.W., Einhorn T.A., Bhandari M. Electrical stimulation for long-bone fracture-healing: a meta-analysis of randomized controlled trials. J. Bone Joint Surg. Am. 2008;90(11):2322–2330. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

