Stem canker pathogen Botryosphaeria dothidea inhibits poplar leaf photosynthesis in the early stage of inoculation
- PMID: 36204063
- PMCID: PMC9530914
- DOI: 10.3389/fpls.2022.1008834
Stem canker pathogen Botryosphaeria dothidea inhibits poplar leaf photosynthesis in the early stage of inoculation
Abstract
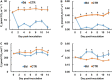
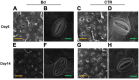
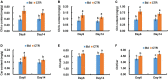
Fungal pathogens can induce canker lesions, wilting, and even dieback in many species. Trees can suffer serious physiological effects from stem cankers. In this study, we investigated the effects of Botryosphaeria dothidea (B. dothidea) on Populus bolleana (P. bolleana) leaves photosynthesis and stomatal responses, when stems were inoculated with the pathogen. To provide experimental and theoretical basis for preventing poplar canker early. One-year-old poplar stems were inoculated with B. dothidea using an epidermal scraping method. In the early stage of B. dothidea inoculation (2-14 days post inoculation, dpi), the gas exchange, stomatal dynamics, hormone content, photosynthetic pigments content, chlorophyll fluorescence parameters, and non-structural carbohydrate (NSC) were evaluated to elucidate the pathophysiological mechanism of B. dothidea inhibiting photosynthesis. Compared with the control groups, B. dothidea noteworthily inhibited the net photosynthetic rate (P n), stomatal conductance (G s), intercellular CO2 concentration (C i), transpiration rate (T r), and other photosynthetic parameters of poplar leaves, but stomatal limit value (L s) increased. Consistent with the above results, B. dothidea also reduced stomatal aperture and stomatal opening rate. In addition, B. dothidea not only remarkably reduced the content of photosynthetic pigments, but also decreased the maximum photochemical efficiency (F v/F m), actual photochemical efficiency (Φ PSII), electron transfer efficiency (ETR), and photochemical quenching coefficient (q P). Furthermore, both chlorophyll and Φ PSII were positively correlated with P n. In summary, the main reason for the abated P n under stem canker pathogen was that B. dothidea not merely inhibited the stomatal opening, but hindered the conversion of light energy, electron transfer and light energy utilization of poplar leaves. In general, the lessened CO2 and P n would reduce the synthesis of photosynthetic products. Whereas, sucrose and starch accumulated in poplar leaves, which may be due to the local damage caused by B. dothidea inoculation in phloem, hindering downward transport of these products.
Keywords: Botryosphaeria dothidea; chlorophyll fluorescence; fungal pathogens; photosynthesis; poplar stem canker; stomatal closure.
Copyright © 2022 Xing, Li, Li, Shen, Li, Zhao and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

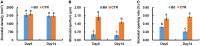




References
-
- Bernal M., Verdaguer D., Badosa J., Abadía A., Llusià J., Peñuelas J., et al. (2015). Effects of enhanced UV radiation and water availability on performance, biomass production and photoprotective mechanisms of Laurus nobilis seedlings. Environ. Exp. Bot. 109 264–275. 10.1016/j.envexpbot.2014.06.016 - DOI
-
- Biggs A. R., Davis D. D., Merrill W. (1983). Histopathology of cankers on Populus caused by Cytospora chrysosperma. Can. J. Bot. 61 563–574. 10.1139/b83-064 - DOI
LinkOut - more resources
Full Text Sources

