Thyroid-related adverse events induced by immune checkpoint inhibitors
- PMID: 36204105
- PMCID: PMC9530140
- DOI: 10.3389/fendo.2022.1010279
Thyroid-related adverse events induced by immune checkpoint inhibitors
Abstract
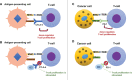
Immune checkpoint inhibitors, namely anti-CTLA-4, anti-PD-1 and anti-PD-L1 monoclonal antibodies, have emerged in the last decade as a novel form of cancer treatment, promoting increased survival in patients. As they tamper with the immune response in order to destroy malignant cells, a new type of adverse reactions has emerged, known as immune-related adverse events (irAEs), which frequently target the endocrine system, especially the thyroid and hypophysis. Thyroid irAEs include hyperthyroidism, thyrotoxicosis, hypothyroidism and a possibly life-threatening condition known as the "thyroid storm". Early prediction of occurrence and detection of the thyroid irAEs should be a priority for the clinician, in order to avoid critical situations. Moreover, they are recently considered both a prognostic marker and a means of overseeing treatment response, since they indicate an efficient activation of the immune system. Therefore, a multidisciplinary approach including both oncologists and endocrinologists is recommended when immune checkpoint inhibitors are used in the clinic.
Keywords: CTLA-4; PD-1; PD-L1; cancer treatment; endocrine; immune checkpoint inhibitors; immune-related adverse events; thyroid.
Copyright © 2022 Chera, Stancu and Bucur.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
New insight in endocrine-related adverse events associated to immune checkpoint blockade.Best Pract Res Clin Endocrinol Metab. 2020 Jan;34(1):101370. doi: 10.1016/j.beem.2019.101370. Epub 2019 Dec 11. Best Pract Res Clin Endocrinol Metab. 2020. PMID: 31983543 Review.
-
Thyroid disorders induced by checkpoint inhibitors.Rev Endocr Metab Disord. 2018 Dec;19(4):325-333. doi: 10.1007/s11154-018-9463-2. Rev Endocr Metab Disord. 2018. PMID: 30242549 Review.
-
PD-L1 Inhibitor-Induced Thyroiditis Is Associated with Better Overall Survival in Cancer Patients.Thyroid. 2020 Feb;30(2):177-184. doi: 10.1089/thy.2019.0250. Epub 2020 Jan 9. Thyroid. 2020. PMID: 31813343 Free PMC article.
-
How can we manage the cardiac toxicity of immune checkpoint inhibitors?Expert Opin Drug Saf. 2021 Jun;20(6):685-694. doi: 10.1080/14740338.2021.1906860. Epub 2021 Apr 1. Expert Opin Drug Saf. 2021. PMID: 33749484 Review.
-
Thyroid Immune-related Adverse Events Following Immune Checkpoint Inhibitor Treatment.J Clin Endocrinol Metab. 2021 Aug 18;106(9):e3704-e3713. doi: 10.1210/clinem/dgab263. J Clin Endocrinol Metab. 2021. PMID: 33878162
Cited by
-
Immune checkpoint inhibitor-induced hypothyroidism predicts treatment response in Japanese subjects.Front Endocrinol (Lausanne). 2023 Jul 31;14:1221723. doi: 10.3389/fendo.2023.1221723. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37583431 Free PMC article.
-
Immune-related adverse events of immune checkpoint inhibitors: a review.Front Immunol. 2023 May 25;14:1167975. doi: 10.3389/fimmu.2023.1167975. eCollection 2023. Front Immunol. 2023. PMID: 37304306 Free PMC article. Review.
-
Case Report: Development of severe inflammatory orbitopathy after immune checkpoint inhibitor initiation.Front Ophthalmol (Lausanne). 2025 Jun 3;5:1574643. doi: 10.3389/fopht.2025.1574643. eCollection 2025. Front Ophthalmol (Lausanne). 2025. PMID: 40529668 Free PMC article.
-
Immunotherapy as a Turning Point in the Treatment of Melanoma Brain Metastases.Discoveries (Craiova). 2023 Jun 30;11(2):e169. doi: 10.15190/d.2023.8. eCollection 2023 Apr-Jun. Discoveries (Craiova). 2023. PMID: 37583899 Free PMC article. Review.
-
Associations Between Pre-Existing Cardiovascular Disease and Survival in Patients on Immune Checkpoint Inhibitor Therapy.Cancer Med. 2025 May;14(9):e70846. doi: 10.1002/cam4.70846. Cancer Med. 2025. PMID: 40296380 Free PMC article.
References
-
- FDA . FDA Approves yervoy for late-stage melanoma (2011). Available at: https://www.webmd.com/melanoma-skin-cancer/news/20110325/fda-approves-ne... (Accessed August 19, 2022).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

