Versatile nanorobot hand biosensor for specific capture and ultrasensitive quantification of viral nanoparticles
- PMID: 36204214
- PMCID: PMC9531290
- DOI: 10.1016/j.mtbio.2022.100444
Versatile nanorobot hand biosensor for specific capture and ultrasensitive quantification of viral nanoparticles
Abstract
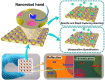
Accurate determination of the concentration and viability of the viral vaccine vectors is urgently needed for preventing the spread of the viral infections, but also supporting the development and assessment of recombinant virus-vectored vaccines. Herein, we describe a nanoplasmonic biosensor with nanoscale robot hand structure (Nano RHB) for the rapid, direct, and specific capture and quantification of adenovirus particles. The nanorobot allows simple operation in practical applications, such as real-time monitoring of vaccine quantity and quality, and evaluation of vaccine viability. Modification of the Nano RHB with branched gold nanostructures allow rapid and efficient assessment of human adenovirus viability, with ultrahigh detection sensitivity of only 100 copies/mL through one-step sandwich method. Nano RHB detection results were consistent with those from the gold standard median tissue culture infectious dose and real-time polymerase chain reaction assays. Additionally, the Nano RHB platform showed high detection specificity for different types of viral vectors and pseudoviruses. Altogether, these results demonstrate that the Nano RHB platform is a promising tool for efficient and ultrasensitive assessment of vaccines and gene delivery vectors.
Keywords: Biosensor; Label-free detection; Nanoparticles; Surface plasmon resonance; Virus vector.
© 2022 Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







Similar articles
-
Highly sensitive dendrimer-based nanoplasmonic biosensor for drug allergy diagnosis.Biosens Bioelectron. 2015 Apr 15;66:115-23. doi: 10.1016/j.bios.2014.10.081. Epub 2014 Nov 13. Biosens Bioelectron. 2015. PMID: 25460891
-
Ultrasensitive early detection of insulin antibody employing novel electrochemical nano-biosensor based on controllable electro-fabrication process.Talanta. 2022 Feb 1;238(Pt 1):122947. doi: 10.1016/j.talanta.2021.122947. Epub 2021 Oct 14. Talanta. 2022. PMID: 34857352
-
Detection and Quantification of Label-Free Infectious Adenovirus Using a Switch-On Cell-Based Fluorescent Biosensor.ACS Sens. 2019 Jun 28;4(6):1654-1661. doi: 10.1021/acssensors.9b00489. Epub 2019 Jun 5. ACS Sens. 2019. PMID: 31117363
-
Large-scale adenovirus and poxvirus-vectored vaccine manufacturing to enable clinical trials.Biotechnol J. 2015 May;10(5):741-7. doi: 10.1002/biot.201400390. Epub 2015 Apr 24. Biotechnol J. 2015. PMID: 25914340 Review.
-
New viral vectors for infectious diseases and cancer.Semin Immunol. 2020 Aug;50:101430. doi: 10.1016/j.smim.2020.101430. Epub 2020 Nov 29. Semin Immunol. 2020. PMID: 33262065 Review.
Cited by
-
MXene-Graphene Oxide Heterostructured Films for Enhanced Metasurface Plasmonic Biosensing in Continuous Glucose Monitoring.Adv Sci (Weinh). 2025 Jan;12(4):e2410376. doi: 10.1002/advs.202410376. Epub 2024 Nov 21. Adv Sci (Weinh). 2025. PMID: 39569760 Free PMC article.
-
Advancements in Micro/Nanorobots in Medicine: Design, Actuation, and Transformative Application.ACS Omega. 2025 Feb 4;10(6):5214-5250. doi: 10.1021/acsomega.4c09806. eCollection 2025 Feb 18. ACS Omega. 2025. PMID: 39989765 Free PMC article. Review.
-
Treatment of lung diseases via nanoparticles and nanorobots: Are these viable alternatives to overcome current treatments?Mater Today Bio. 2025 Feb 26;31:101616. doi: 10.1016/j.mtbio.2025.101616. eCollection 2025 Apr. Mater Today Bio. 2025. PMID: 40124344 Free PMC article. Review.
-
Nanotechnology-Assisted Biosensors for the Detection of Viral Nucleic Acids: An Overview.Biosensors (Basel). 2023 Jan 30;13(2):208. doi: 10.3390/bios13020208. Biosensors (Basel). 2023. PMID: 36831973 Free PMC article. Review.
-
Nanozyme-Catalyzed Metasurface Plasmon Sensor-Based Portable Ultrasensitive Optical Quantification Platform for Cancer Biomarker Screening.Adv Sci (Weinh). 2023 Aug;10(24):e2301658. doi: 10.1002/advs.202301658. Epub 2023 Jun 26. Adv Sci (Weinh). 2023. PMID: 37358326 Free PMC article.
References
-
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J.a., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. - DOI - PMC - PubMed
-
- Kraemer M.U.G., Yang C.-H., Gutierrez B., Wu C.-H., Klein B., Pigott D.M., Plessis L.d., Faria N.R., Li R., Hanage W.P., Brownstein J.S., Layan M., Vespignani A., Tian H., Dye C., Pybus O.G., Scarpino S.V. The effect of human mobility and control measures on the covid-19 epidemic in China. Science. 2020;368:493–497. doi: 10.1126/science.abb4218. - DOI - PMC - PubMed
-
- Afkhami S., D'Agostino M.R., Zhang A., Stacey H.D., Marzok A., Kang A., Singh R., Bavananthasivam J., Ye G., Luo X., Wang F., Ang J.C., Zganiacz A., Sankar U., Kazhdan N., Koenig J.F.E., Phelps A., Gameiro S.F., Tang S., Jordana M., Wan Y., Mossman K.L., Jeyanathan M., Gillgrass A., Medina M.F.C., Smaill F., Lichty B.D., Miller M.S., Xing Z. 2022. Respiratory Mucosal Delivery of Next-Generation Covid-19 Vaccine Provides Robust Protection against Both Ancestral and Variant Strains of Sars-Cov-2, Cell. - DOI - PMC - PubMed
-
- Capone S., Raggioli A., Gentile M., Battella S., Lahm A., Sommella A., Contino A.M., Urbanowicz R.A., Scala R., Barra F., Leuzzi A., Lilli E., Miselli G., Noto A., Ferraiuolo M., Talotta F., Tsoleridis T., Castilletti C., Matusali G., Colavita F., Lapa D., Meschi S., Capobianchi M., Soriani M., Folgori A., Ball J.K., Colloca S., Vitelli A. Immunogenicity of a new gorilla adenovirus vaccine candidate for covid-19. Mol. Ther. 2021;29:2412–2423. doi: 10.1016/j.ymthe.2021.04.022. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

