Molecular Mechanisms of Spawning Habits for the Adaptive Radiation of Endemic East Asian Cyprinid Fishes
- PMID: 36204246
- PMCID: PMC9513835
- DOI: 10.34133/2022/9827986
Molecular Mechanisms of Spawning Habits for the Adaptive Radiation of Endemic East Asian Cyprinid Fishes
Abstract
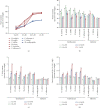
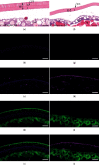
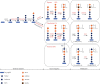
Despite the widespread recognition of adaptive radiation as a driver of speciation, the mechanisms by which natural selection generates new species are incompletely understood. The evolutionary radiation of endemic East Asian cyprinids has been proposed as evolving through a change in spawning habits, involving a transition from semibuoyant eggs to adhesive eggs in response to crosslinked river-lake system formation. Here, we investigated the molecular mechanisms that underpin this radiation, associated with egg hydration and adhesiveness. We demonstrated that semibuoyant eggs enhance hydration by increasing the degradation of yolk protein and accumulation of Ca2+ and Mg2+ ions, while adhesive eggs improve adhesiveness and hardness of the egg envelope by producing an adhesive layer and a unique 4th layer to the egg envelope. Based on multiomics analyses and verification tests, we showed that during the process of adaptive radiation, adhesive eggs downregulated the "vitellogenin degradation pathway," "zinc metalloprotease pathway," and "ubiquitin-proteasome pathway" and the pathways of Ca2+ and Mg2+ active transport to reduce their hydration. At the same time, adhesive eggs upregulated the crosslinks of microfilament-associated proteins and adhesive-related proteins, the hardening-related proteins of the egg envelope, and the biosynthesis of glycosaminoglycan in the ovary to generate adhesiveness. These findings illustrate the novel molecular mechanisms associated with hydration and adhesiveness of freshwater fish eggs and identify critical molecular mechanisms involved in the adaptive radiation of endemic East Asian cyprinids. We propose that these key egg attributes may function as "magic traits" in this adaptive radiation.
Copyright © 2022 Feng Chen et al.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this article.
Figures





References
-
- Darwin C. On the Origin of Species . John Murray; 1859.
-
- Schluter D. The Ecology of Adaptive Radiation . Oxford: OUP; 2000.
LinkOut - more resources
Full Text Sources
Miscellaneous

