Extended effects of a wearable sensory prosthesis on gait, balance function and falls after 26 weeks of use in persons with peripheral neuropathy and high fall risk-The walk2Wellness trial
- PMID: 36204554
- PMCID: PMC9531134
- DOI: 10.3389/fnagi.2022.931048
Extended effects of a wearable sensory prosthesis on gait, balance function and falls after 26 weeks of use in persons with peripheral neuropathy and high fall risk-The walk2Wellness trial
Abstract
Background: We recently reported that individuals with impaired plantar sensation and high fall risk due to sensory peripheral neuropathy (PN) improved gait and balance function following 10 weeks of use of Walkasins®, a wearable lower limb sensory prosthesis that provides directional specific mechanical tactile stimuli related to plantar pressure measurements during standing and walking (RxFunction Inc., Eden Prairie, MN, United States). Here, we report 26-week outcomes and compare pre- and in-study fall rates. We expected improvements in outcomes and reduced fall rates reported after 10 weeks of use to be sustained.
Materials and methods: Participants had clinically diagnosed PN with impaired plantar sensation, high fall risk (Functional Gait Assessment, FGA score < 23) and ability to sense tactile stimuli above the ankle at the location of the device. Additional outcomes included 10 m Gait Speed, Timed Up and Go (TUG), Four-Stage Balance Test, and self-reported outcomes, including Activities-Specific Balance Confidence scale and Vestibular Disorders Activities of Daily Living Scale. Participants tracked falls using a calendar.
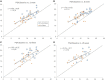
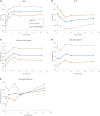
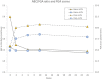
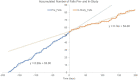
Results: We assessed falls and self-reported outcomes from 44 individuals after 26 weeks of device use; 30 of them conducted in-person testing of clinical outcomes. Overall, improvements in clinical outcomes seen at 10 weeks of use remained sustained at 26 weeks with statistically significant increases compared to baseline seen in FGA scores (from 15.0 to 19.2), self-selected gait speed (from 0.89 to 0.97 m/s), and 4-Stage Balance Test (from 25.6 to 28.4 s), indicating a decrease in fall risk. Non-significant improvements were observed in TUG and fast gait speed. Overall, 39 falls were reported; 31 of them did not require medical treatment and four caused severe injury. Participants who reported falls over 6 months prior to the study had a 43% decrease in fall rate during the study as compared to self-report 6-month pre-study (11.8 vs. 6.7 falls/1000 patient days, respectively, p < 0.004), similar to the 46% decrease reported after 10 weeks of use.
Conclusion: A wearable sensory prosthesis can improve outcomes of gait and balance function and substantially decreases incidence of falls during long-term use. The sustained long-term benefits in clinical outcomes reported here lessen the likelihood that improvements are placebo effects.
Clinical trial registration: ClinicalTrials.gov, identifier #NCT03538756.
Keywords: balance; clinical trial; falls; gait speed; neuromodulation; peripheral neuropathy; sensory prosthesis; wearable.
Copyright © 2022 Oddsson, Bisson, Cohen, Iloputaife, Jacobs, Kung, Lipsitz, Manor, McCracken, Rumsey, Wrisley and Koehler-McNicholas.
Conflict of interest statement
LO is an inventor of the technology, Co-Founder of RxFunction Inc., a shareholder in the company, serves on its Board of Directors, and is an employee of the company. LJ and YR are employees and shareholders of RxFunction Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
The Effects of a Wearable Sensory Prosthesis on Gait and Balance Function After 10 Weeks of Use in Persons With Peripheral Neuropathy and High Fall Risk - The walk2Wellness Trial.Front Aging Neurosci. 2020 Nov 9;12:592751. doi: 10.3389/fnagi.2020.592751. eCollection 2020. Front Aging Neurosci. 2020. PMID: 33240077 Free PMC article.
-
Long-Term Use of a Sensory Prosthesis Improves Function in a Patient With Peripheral Neuropathy: A Case Report.Front Neurol. 2021 Jun 23;12:655963. doi: 10.3389/fneur.2021.655963. eCollection 2021. Front Neurol. 2021. PMID: 34248817 Free PMC article.
-
Erratum.Mult Scler. 2016 Oct;22(12):NP9-NP11. doi: 10.1177/1352458515585718. Epub 2015 Jun 3. Mult Scler. 2016. PMID: 26041800
-
Exercise for improving outcomes after osteoporotic vertebral fracture.Cochrane Database Syst Rev. 2019 Jul 5;7(7):CD008618. doi: 10.1002/14651858.CD008618.pub3. Cochrane Database Syst Rev. 2019. PMID: 31273764 Free PMC article.
-
Exercise for preventing falls in older people living in the community.Cochrane Database Syst Rev. 2019 Jan 31;1(1):CD012424. doi: 10.1002/14651858.CD012424.pub2. Cochrane Database Syst Rev. 2019. PMID: 30703272 Free PMC article.
Cited by
-
TRPML1 ion channel promotes HepaRG cell differentiation under simulated microgravity conditions.NPJ Microgravity. 2025 Mar 15;11(1):9. doi: 10.1038/s41526-025-00461-4. NPJ Microgravity. 2025. PMID: 40089547 Free PMC article.
-
Wearable non-invasive neuroprosthesis for targeted sensory restoration in neuropathy.Nat Commun. 2024 Dec 30;15(1):10840. doi: 10.1038/s41467-024-55152-7. Nat Commun. 2024. PMID: 39738088 Free PMC article.
-
Six month lower-leg mechanical tactile sensory stimulation alters functional network connectivity associated with improved gait in older adults with peripheral neuropathy - A pilot study.Front Aging Neurosci. 2022 Nov 3;14:1027242. doi: 10.3389/fnagi.2022.1027242. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36408098 Free PMC article.
References
-
- Algina J., Keselman H. J., Penfield R. D. (2005). Effect sizes and their intervals: The two-level repeated measures case. Educ. Psychol. Meas. 65 241–258. 10.1177/0013164404268675 - DOI
-
- Bao T., Carender W. J., Kinnaird C., Barone V. J., Peethambaran G., Whitney S. L., et al. (2018). Effects of long-term balance training with vibrotactile sensory augmentation among community-dwelling healthy older adults: A randomized preliminary study. J. Neuroeng. Rehabil. 15:5. 10.1186/s12984-017-0339-6 - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

