Selexipag-based triple combination therapy improves prognosis in Chinese pulmonary arterial hypertension patients
- PMID: 36204579
- PMCID: PMC9530145
- DOI: 10.3389/fcvm.2022.991586
Selexipag-based triple combination therapy improves prognosis in Chinese pulmonary arterial hypertension patients
Abstract
Aim: Selexipag is an oral selective prostacyclin receptor agonist approved for treatment of patients with pulmonary arterial hypertension (PAH). In the present study, we aim to assess the safety and efficacy of selexipag in triple combination therapy with endothelial receptor antagonists (ERAs) and PDE5is for Chinese PAH patients.
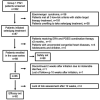
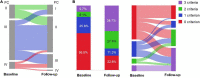
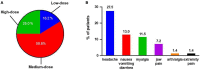
Methods and results: A single center retrospective study was performed on group 1 PAH patients (n = 68) initiating triple combination therapy with selexipag from 1 February 2020 to 31 August 2021 in Qilu Hospital of Shandong University (Shandong, China). Adolescents, children, and PAH patients with unrepaired congenital heart disease were excluded. The French pulmonary hypertension network (FPHN) non-invasive risk assessment, echocardiogram parameters, and clinical data, including tolerability, safety, and death/hospitalization events associated with PAH, were collected. Of the 68 patients, 31 (45.6%) patients had tolerable side effects while only a single patient discontinued selexipag due to severe diarrhea. In the analysis of the efficacy set of 62 patients, the median selexipag treatment time from selexipag initiation to last risk assessment was 27 (21, 33) weeks. Compared to baseline parameters, the percentage of WHO FC III/IV decreased from 77.4% (48) to 24.2% (15) (p = 0.000), median 6-min walk distance (6MWD) increased 82 m [from 398 (318, 450) to 480 (420, 506) m; p = 0.000], and NT-proBNP levels decreased from 1,216 (329, 2,159) to 455 (134, 1,678) pg/mL (p = 0.007). Patients who improved to three low-risk criteria increased from 9.7 to 38.7%. Right ventricular diameter (RV) diameter also decreased and was accompanied by an improved tricuspid annular plane systolic excursion (TAPSE). Patients transitioning from subcutaneous treprostinil to selexipag continued to show improvements in WHO FC, 6MWD (404 ± 94 vs. 383 ± 127 m) and NT-proBNP levels (2,319 ± 2,448 vs. 2,987 ± 3,770 pg/mL). Finally, the 1-year event free survival rate was 96.7% for patients initiating the triple combination therapy within 3 years of PAH diagnosis.
Conclusion: Triple combination therapy with selexipag was safe and effective in Chinese PAH patients, which was confirmed by acceptable tolerability, and improved exercise capacity, right heart function, risk assessment, and prognosis.
Keywords: pulmonary arterial hypertension; risk assessment; selexipag; tolerability; triple combination.
Copyright © 2022 Cui, Lu, Zhang, Qie, Li, Li, Liu and Ji.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Simonneau G, Barst RJ, Galie N, Naeije R, Rich S, Bourge RC, et al. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. (2002) 165:800–4. 10.1164/ajrccm.165.6.2106079 - DOI - PubMed
-
- Hoeper MM, McLaughlin VV, Barbera JA, Frost AE, Ghofrani HA, Peacock AJ, et al. Initial combination therapy with ambrisentan and tadalafil and mortality in patients with pulmonary arterial hypertension: a secondary analysis of the results from the randomised, controlled AMBITION study. Lancet Respir Med. (2016) 4:894–901. 10.1016/S2213-2600(16)30307-1 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

