Controlled human infectious models, a path forward in uncovering immunological correlates of protection: Lessons from enteric fevers studies
- PMID: 36204615
- PMCID: PMC9530043
- DOI: 10.3389/fmicb.2022.983403
Controlled human infectious models, a path forward in uncovering immunological correlates of protection: Lessons from enteric fevers studies
Abstract
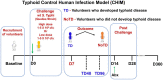
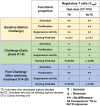
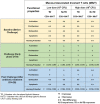
Enteric infectious diseases account for more than a billion disease episodes yearly worldwide resulting in approximately 2 million deaths, with children under 5 years old and the elderly being disproportionally affected. Enteric pathogens comprise viruses, parasites, and bacteria; the latter including pathogens such as Salmonella [typhoidal (TS) and non-typhoidal (nTS)], cholera, Shigella and multiple pathotypes of Escherichia coli (E. coli). In addition, multi-drug resistant and extensively drug-resistant (XDR) strains (e.g., S. Typhi H58 strain) of enteric bacteria are emerging; thus, renewed efforts to tackle enteric diseases are required. Many of these entero-pathogens could be controlled by oral or parenteral vaccines; however, development of new, effective vaccines has been hampered by lack of known immunological correlates of protection (CoP) and limited knowledge of the factors contributing to protective responses. To fully comprehend the human response to enteric infections, an invaluable tool that has recently re-emerged is the use of controlled human infection models (CHIMs) in which participants are challenged with virulent wild-type (wt) organisms. CHIMs have the potential to uncover immune mechanisms and identify CoP to enteric pathogens, as well as to evaluate the efficacy of therapeutics and vaccines in humans. CHIMs have been used to provide invaluable insights in the pathogenesis, host-pathogen interaction and evaluation of vaccines. Recently, several Oxford typhoid CHIM studies have been performed to assess the role of multiple cell types (B cells, CD8+ T, Tregs, MAIT, Monocytes and DC) during S. Typhi infection. One of the key messages that emerged from these studies is that baseline antigen-specific responses are important in that they can correlate with clinical outcomes. Additionally, volunteers who develop typhoid disease (TD) exhibit higher levels and more activated cell types (e.g., DC and monocytes) which are nevertheless defective in discrete signaling pathways. Future critical aspects of this research will involve the study of immune responses to enteric infections at the site of entry, i.e., the intestinal mucosa. This review will describe our current knowledge of immunity to enteric fevers caused by S. Typhi and S. Paratyphi A, with emphasis on the contributions of CHIMs to uncover the complex immunological responses to these organisms and provide insights into the determinants of protective immunity.
Keywords: challenge human infection model; correlates of protection; enteric diseases; immunity; typhoid; vaccine.
Copyright © 2022 Sztein and Booth.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Betts M. R., Brenchley J. M., Price D. A., De Rosa S. C., Douek D. C., Roederer M., et al. (2003). Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods 281, 65–78. doi: 10.1016/S0022-1759(03)00265-5, PMID: - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

