Neural and Müller glial adaptation of the retina to photoreceptor degeneration
- PMID: 36204825
- PMCID: PMC9700092
- DOI: 10.4103/1673-5374.354511
Neural and Müller glial adaptation of the retina to photoreceptor degeneration
Abstract
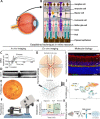
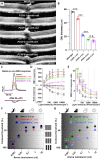
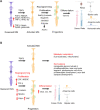
The majority of inherited retinal degenerative diseases and dry age-related macular degeneration are characterized by decay of the outer retina and photoreceptors, which leads to progressive loss of vision. The inner retina, including second- and third-order retinal neurons, also shows aberrant structural changes at all stages of degeneration. Müller glia, the major glial cells maintain retinal homeostasis, activating and rearranging immediately in response to photoreceptor stress. These phenomena are collectively known as retinal remodeling and are anatomically well described, but their impact on visual function is less well characterized. Retinal remodeling has traditionally been considered a detrimental chain of events that decreases visual function. However, emerging evidence from functional assays suggests that remodeling could also be a part of a survival mechanism wherein the inner retina responds plastically to outer retinal degeneration. The visual system´s first synapses between the photoreceptors and bipolar cells undergo rewiring and functionally compensate to maintain normal signal output to the brain. Distinct classes of retinal ganglion cells remain even after the massive loss of photoreceptors. Müller glia possess the regenerative potential for retinal recovery and possibly exert adaptive transcriptional changes in response to neuronal loss. These types of homeostatic changes could potentially explain the well-maintained visual function observed in patients with inherited retinal degenerative diseases who display prominent anatomic retinal pathology. This review will focus on our current understanding of retinal neuronal and Müller glial adaptation for the potential preservation of retinal activity during photoreceptor degeneration. Targeting retinal self-compensatory responses could help generate universal strategies to delay sensory disease progression.
Keywords: Müller glia; bipolar cells; electroretinography; photoreceptors; plasticity; retinal degeneration; retinal ganglion cells; retinal neuron; retinal remodeling.
Conflict of interest statement
None
Figures
References
-
- Ait-Ali N, Fridlich R, Millet-Puel G, Clerin E, Delalande F, Jaillard C, Blond F, Perrocheau L, Reichman S, Byrne LC, Olivier-Bandini A, Bellalou J, Moyse E, Bouillaud F, Nicol X, Dalkara D, van Dorsselaer A, Sahel JA, Leveillard T. Rod-derived cone viability factor promotes cone survival by stimulating aerobic glycolysis. Cell. 2015;161:817–832. - PubMed
-
- Aleman TS, LaVail MM, Montemayor R, Ying G, Maguire MM, Laties AM, Jacobson SG, Cideciyan AV. Augmented rod bipolar cell function in partial receptor loss:an ERG study in P23H rhodopsin transgenic and aging normal rats. Vision Res. 2001;41:2779–2797. - PubMed
-
- Barhoum R, Martinez-Navarrete G, Corrochano S, Germain F, Fernandez-Sanchez L, de la Rosa EJ, de la Villa P, Cuenca N. Functional and structural modifications during retinal degeneration in the rd10 mouse. Neuroscience. 2008;155:698–713. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

