Arteriovenous Fistulae in Chronic Kidney Disease and the Heart: Physiological, Histological, and Transcriptomic Characterization of a Novel Rat Model
- PMID: 36205249
- PMCID: PMC9673660
- DOI: 10.1161/JAHA.122.027593
Arteriovenous Fistulae in Chronic Kidney Disease and the Heart: Physiological, Histological, and Transcriptomic Characterization of a Novel Rat Model
Abstract
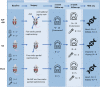
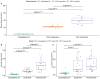
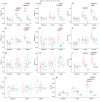
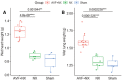
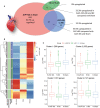
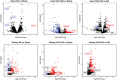
Background Arteriovenous fistulae (AVFs) are the gold standard for vascular access in those requiring hemodialysis but may put an extra hemodynamic stress on the cardiovascular system. The complex interactions between the heart, kidney, and AVFs remain incompletely understood. Methods and Results We characterized a novel rat model of five-sixths partial nephrectomy (NX) and AVFs. NX induced increases in urea, creatinine, and hippuric acid. The addition of an AVF (AVF+NX) further increased urea and a number of uremic toxins such as trimethylamine N-oxide and led to increases in cardiac index, left and right ventricular volumes, and right ventricular mass. Plasma levels of uremic toxins correlated well with ventricular morphology and function. Heart transcriptomes identified altered expression of 8 genes following NX and 894 genes following AVF+NX, whereas 290 and 1431 genes were altered in the kidney transcriptomes, respectively. Gene ontology and Kyoto Encyclopedia of Genes and Genomes analysis revealed gene expression changes related to cell division and immune activation in both organs, suppression of ribosomes and transcriptional activity in the heart, and altered renin-angiotensin signaling as well as chronodisruption in the kidney. All except the latter were worsened in AVF+NX compared with NX. Conclusions Inflammation and organ dysfunction in chronic kidney disease are exacerbated following AVF creation. Furthermore, our study provides important information for the discovery of novel biomarkers and therapeutic targets in the management of cardiorenal syndrome.
Keywords: animal model; arteriovenous fistula; cardiorenal syndrome; chronic kidney disease; heart failure.
Figures





Similar articles
-
The impact of location and patency of the arteriovenous fistula on quality of life of kidney transplant recipients.Ren Fail. 2021 Dec;43(1):113-122. doi: 10.1080/0886022X.2020.1865171. Ren Fail. 2021. PMID: 33397180 Free PMC article.
-
Right Ventricular Enlargement within Months of Arteriovenous Fistula Creation in 2 Hemodialysis Patients.Tex Heart Inst J. 2016 Aug 1;43(4):350-3. doi: 10.14503/THIJ-15-5353. eCollection 2016 Aug. Tex Heart Inst J. 2016. PMID: 27547150 Free PMC article.
-
Factors associated with functional arteriovenous fistula at hemodialysis start and arteriovenous fistula non-use in a single-center cohort.J Vasc Access. 2022 Jul;23(4):558-566. doi: 10.1177/11297298211002574. Epub 2021 Mar 22. J Vasc Access. 2022. PMID: 33752497
-
Arteriovenous fistula in dialysis patients: Factors implicated in early and late AVF maturation failure.Surgeon. 2016 Oct;14(5):294-300. doi: 10.1016/j.surge.2016.02.001. Epub 2016 Mar 15. Surgeon. 2016. PMID: 26988630 Review.
-
The Impact of Arteriovenous Fistulae for Hemodialysis on the Cardiovascular System.Semin Dial. 2016 May;29(3):214-21. doi: 10.1111/sdi.12459. Epub 2016 Jan 12. Semin Dial. 2016. PMID: 26756565 Review.
Cited by
-
Metabolomics Assessment of Volume Overload-Induced Heart Failure and Oxidative Stress in the Kidney.Metabolites. 2023 Nov 20;13(11):1165. doi: 10.3390/metabo13111165. Metabolites. 2023. PMID: 37999260 Free PMC article.
-
The rodent models of arteriovenous fistula.Front Cardiovasc Med. 2024 Jan 18;11:1293568. doi: 10.3389/fcvm.2024.1293568. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 38304139 Free PMC article. Review.
References
-
- Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

