Aeromonas salmonicida binds α2-6 linked sialic acid, which is absent among the glycosphingolipid repertoires from skin, gill, stomach, pyloric caecum, and intestine
- PMID: 36205522
- PMCID: PMC9553145
- DOI: 10.1080/21505594.2022.2132056
Aeromonas salmonicida binds α2-6 linked sialic acid, which is absent among the glycosphingolipid repertoires from skin, gill, stomach, pyloric caecum, and intestine
Abstract
Carbohydrates can both protect against infection and act as targets promoting infection. Mucins are major components of the slimy mucus layer covering the fish epithelia. Mucins can act as decoys for intimate pathogen interaction with the host afforded by binding to glycosphingolipids in the host cell membrane. We isolated and characterized glycosphingolipids from Atlantic salmon skin, gill, stomach, pyloric caeca, and intestine. We characterized the glycosphingolipids using liquid chromatography - mass spectrometry and tandem mass spectrometry and the glycan repertoire was compared with the glycan repertoire of mucins from the same epithelia. We also investigated Aeromonas salmonicida binding using chromatogram and microtiter well based binding assays. We identified 29 glycosphingolipids. All detected acid glycans were of the ganglio-series (unless shorter) and showed a high degree of polysialylation. The non-acid glycans were mostly composed of the neolacto, globo, and ganglio core structures. The glycosphingolipid repertoire differed between epithelia and the proportion of the terminal moieties of the glycosphingolipids did not reflect the terminal moieties on the mucins from the same epithelia. A. salmonicida did not bind the Atlantic salmon glycosphingolipids. Instead, we identified that A. salmonicida binding to sialic acid occurred to α2-6 Neu5Ac but not to α2-3 Neu5Ac. α2-6 Neu5Ac was present on mucins whereas mainly α2-3 Neu5Ac was found on the glycosphingolipids, explaining the difference in A. salmonicida binding ability between these host glycoconjugates. A. salmonicida´s ability to bind to Atlantic salmon mucins, but not the glycosphingolipids, is likely part of the host defence against this pathogen.
Keywords: Aeromonas salmonicida; Atlantic salmon; Glycosphingolipid; bacteria; epithelial surface; glycan; host–pathogen interaction; mucin; mucosa.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


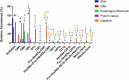


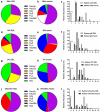

References
-
- Sharba S, Sundh H, Sundell K, et al. Rainbow trout gastrointestinal mucus, mucin production, mucin glycosylation and response to lipopolysaccharide. Fish Shellfish Immunol. 2022;122:181–190. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
