Association Between Vaccination Status and Mortality Among Intubated Patients With COVID-19-Related Acute Respiratory Distress Syndrome
- PMID: 36205996
- PMCID: PMC9547321
- DOI: 10.1001/jamanetworkopen.2022.35219
Association Between Vaccination Status and Mortality Among Intubated Patients With COVID-19-Related Acute Respiratory Distress Syndrome
Abstract
Importance: Although vaccination substantially reduces the risk of severe COVID-19, it is yet unknown whether vaccinated patients who develop COVID-19 and require invasive mechanical ventilation have lower mortality than controls.
Objective: To examine the association between COVID-19 vaccination status and mortality among critically ill patients who require invasive mechanical ventilation owing to acute respiratory distress syndrome (ARDS) related to COVID-19.
Design, setting, and participants: This multicenter cohort study was performed between June 7, 2021, and February 1, 2022, among 265 consecutive adult patients with COVID-19 in academic intensive care units who underwent invasive mechanical ventilation owing to ARDS.
Exposures: Patients in the full vaccination group had completed the primary COVID-19 vaccination series more than 14 days but less than 5 months prior to intubation. This time threshold was chosen because guidelines from the US Centers for Disease Control and Prevention recommend a booster dose beyond that time. The remaining patients (ie, those who were unvaccinated, partially vaccinated, or fully vaccinated <14 days or >5 months before intubation) comprised the control group.
Main outcomes and measures: The primary outcome was time from intubation to all-cause intensive care unit mortality. A Cox proportional hazards regression model including vaccination status, age, comorbid conditions, and baseline Sequential Organ Failure Assessment score on the day of intubation was used.
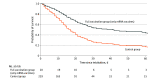
Results: A total of 265 intubated patients (170 men [64.2%]; median age, 66.0 years [IQR, 58.0-76.0 years]; 26 [9.8%] in the full vaccination group) were included in the study. A total of 20 patients (76.9%) in the full vaccination group received the BNT162b2 vaccine, and the remaining 6 (23.1%) received the ChAdOx1 nCoV-19 vaccine. Patients in the full vaccination group were older (median age, 72.5 years [IQR, 62.8-80.0 years] vs 66.0 years [IQR, 57.0-75.0 years]) and more likely to have comorbid conditions (24 of 26 [92.3%] vs 160 of 239 [66.9%]), including malignant neoplasm (6 of 26 [23.1%] vs 18 of 239 [7.5%]), than those in the control group. Full vaccination status was significantly associated with lower mortality compared with controls (16 of 26 patients [61.5%] died in the full vaccination group vs 163 of 239 [68.2%] in the control group; hazard ratio, 0.55 [95% CI, 0.32-0.94]; P = .03).
Conclusions and relevance: In this cohort study, full vaccination status was associated with lower mortality compared with controls, which suggests that vaccination might be beneficial even among patients who were intubated owing to COVID-19-related ARDS. These results may inform discussions with families about prognosis.
Conflict of interest statement
Figures

References
-
- Cohen MJ, Oster Y, Moses AE, Spitzer A, Benenson S; Israeli-Hospitals 4th Vaccine Working Group . Association of receiving a fourth dose of the BNT162b vaccine with SARS-CoV-2 infection among health care workers in Israel. JAMA Netw Open. 2022;5(8):e2224657. doi:10.1001/jamanetworkopen.2022.24657 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

