Multiple COVID-19 vaccine doses in CLL and MBL improve immune responses with progressive and high seroconversion
- PMID: 36206503
- PMCID: PMC9550283
- DOI: 10.1182/blood.2022017814
Multiple COVID-19 vaccine doses in CLL and MBL improve immune responses with progressive and high seroconversion
Abstract
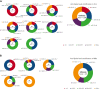
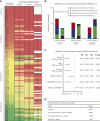
Patients with chronic lymphocytic leukemia (CLL) or monoclonal B-lymphocytosis (MBL) have impaired response to COVID-19 vaccination. A total of 258 patients (215 with CLL and 43 with MBL) had antispike antibody levels evaluable for statistical analysis. The overall seroconversion rate in patients with CLL was 94.2% (antispike antibodies ≥50 AU/mL) and 100% in patients with MBL after multiple vaccine doses. After 3 doses (post-D3) in 167 patients with CLL, 73.7% were seropositive, 17.4% had antispike antibody levels between 50 and 999 AU/mL, and 56.3% had antispike antibody levels ≥1000 AU/mL, with a median rise from 144.6 to 1800.7 AU/mL. Of patients who were seronegative post-D2, 39.7% seroconverted post-D3. For those who then remained seronegative after their previous dose, seroconversion occurred in 40.6% post-D4, 46.2% post-D5, 16.7% post-D6, and 0% after D7 or D8. After seroconversion, most had a progressive increase in antispike antibody levels. Neutralization was associated with higher antispike antibody levels, more vaccine doses, and earlier severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants; neutralizing antibody against early clade D614G was detected in 65.3%, against Delta in 52.0%, and against Omicron in 36.5%. SARS-CoV-2-specific T-cell production of interferon γ and interleukin 2 occurred in 73.9% and 60.9%, respectively, of 23 patients tested. After multiple vaccine doses, by multivariate analysis, immunoglobulin M ≥0.53 g/L, immunoglobulin subclass G3 ≥0.22 g/L and absence of current CLL therapy were independent predictors of positive serological responses. Multiple sequential COVID-19 vaccination significantly increased seroconversion and antispike antibody levels in patients with CLL or MBL.
© 2022 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures




Comment in
-
SARS-CoV-2 vaccination in CLL: how often is enough?Blood. 2022 Dec 22;140(25):2655-2657. doi: 10.1182/blood.2022018586. Blood. 2022. PMID: 36548017 Free PMC article. No abstract available.
References
-
- Andersen MA, Vojdeman FJ, Andersen MK, et al. Hypogammaglobulinemia in newly diagnosed chronic lymphocytic leukemia is a predictor of early death. Leuk Lymphoma. 2016;57(7):1592–1599. - PubMed
-
- Crassini KR, Zhang E, Balendran S, et al. Humoral immune failure defined by immunoglobulin class and immunoglobulin G subclass deficiency is associated with shorter treatment-free and overall survival in chronic lymphocytic leukaemia. Br J Haematol. 2018;181(1):97–101. - PubMed
-
- Freeman JA, Crassini KR, Best OG, et al. Immunoglobulin G subclass deficiency and infection risk in 150 patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2013;54(1):99–104. - PubMed
-
- Forconi F, Moss P. Perturbation of the normal immune system in patients with CLL. Blood. 2015;126(5):573–581. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

