The fatal trajectory of pulmonary COVID-19 is driven by lobular ischemia and fibrotic remodelling
- PMID: 36206625
- PMCID: PMC9535314
- DOI: 10.1016/j.ebiom.2022.104296
The fatal trajectory of pulmonary COVID-19 is driven by lobular ischemia and fibrotic remodelling
Abstract
Background: COVID-19 is characterized by a heterogeneous clinical presentation, ranging from mild symptoms to severe courses of disease. 9-20% of hospitalized patients with severe lung disease die from COVID-19 and a substantial number of survivors develop long-COVID. Our objective was to provide comprehensive insights into the pathophysiology of severe COVID-19 and to identify liquid biomarkers for disease severity and therapy response.
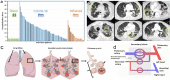
Methods: We studied a total of 85 lungs (n = 31 COVID autopsy samples; n = 7 influenza A autopsy samples; n = 18 interstitial lung disease explants; n = 24 healthy controls) using the highest resolution Synchrotron radiation-based hierarchical phase-contrast tomography, scanning electron microscopy of microvascular corrosion casts, immunohistochemistry, matrix-assisted laser desorption ionization mass spectrometry imaging, and analysis of mRNA expression and biological pathways. Plasma samples from all disease groups were used for liquid biomarker determination using ELISA. The anatomic/molecular data were analyzed as a function of patients' hospitalization time.
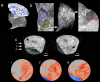
Findings: The observed patchy/mosaic appearance of COVID-19 in conventional lung imaging resulted from microvascular occlusion and secondary lobular ischemia. The length of hospitalization was associated with increased intussusceptive angiogenesis. This was associated with enhanced angiogenic, and fibrotic gene expression demonstrated by molecular profiling and metabolomic analysis. Increased plasma fibrosis markers correlated with their pulmonary tissue transcript levels and predicted disease severity. Plasma analysis confirmed distinct fibrosis biomarkers (TSP2, GDF15, IGFBP7, Pro-C3) that predicted the fatal trajectory in COVID-19.
Interpretation: Pulmonary severe COVID-19 is a consequence of secondary lobular microischemia and fibrotic remodelling, resulting in a distinctive form of fibrotic interstitial lung disease that contributes to long-COVID.
Funding: This project was made possible by a number of funders. The full list can be found within the Declaration of interests / Acknowledgements section at the end of the manuscript.
Keywords: Biomarkers; COVID-19; Fibrogenesis; Intussusceptive angiogenesis.
Copyright © 2022 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests HHK received fees for lectures and consultations from Roche Pharma AG, Novartis, AstraZeneca, Genomic Health, Pfizer, and Amgen, all outside the present study. MMH received fees for lectures and consultations from Acceleron, Actelion, Bayer, GSK, Janssen, MSD, and Pfizer, all outside the present study. TW declares funding by the German Ministry of Research and Education. MAK and DJL declare the possession of “Nordic Bioscience” stock options. BS received fees for lectures from Boehringer Ingelheim. The other authors have no potential conflicts of interest to report.
Figures



Comment in
-
Endothelial cells are major players in SARS-CoV-2-related acute respiratory distress syndrome.EBioMedicine. 2022 Dec;86:104328. doi: 10.1016/j.ebiom.2022.104328. Epub 2022 Nov 3. EBioMedicine. 2022. PMID: 36335670 Free PMC article. No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

