Behaviourally modulated hippocampal theta oscillations in the ferret persist during both locomotion and immobility
- PMID: 36207304
- PMCID: PMC9547075
- DOI: 10.1038/s41467-022-33507-2
Behaviourally modulated hippocampal theta oscillations in the ferret persist during both locomotion and immobility
Abstract
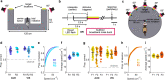
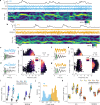
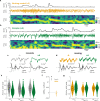
Theta oscillations are a hallmark of hippocampal activity across mammals and play a critical role in many hippocampal models of memory and spatial navigation. To reconcile the cross-species differences observed in the presence and properties of theta, we recorded hippocampal local field potentials in rats and ferrets during auditory and visual localisation tasks designed to vary locomotion and sensory attention. Here, we show that theta oscillations occur during locomotion in both ferrets and rats, however during periods of immobility, theta oscillations persist in the ferret, contrasting starkly with the switch to large irregular activity (LIA) in the rat. Theta during immobility in the ferret is identified as analogous to Type 2 theta that has been observed in rodents due to its sensitivity to atropine, and is modulated by behavioural state with the strongest theta observed during reward epochs. These results demonstrate that even under similar behavioural conditions, differences exist between species in the relationship between theta and behavioural state.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Behavioral correlates of hippocampal type 2 theta in the rat.Physiol Behav. 1987;39(4):513-9. doi: 10.1016/0031-9384(87)90382-9. Physiol Behav. 1987. PMID: 3575499
-
Cross-strata co-occurrence of ripples with theta-frequency oscillations in the hippocampus of foraging rats.J Physiol. 2024 May;602(10):2315-2341. doi: 10.1113/JP284629. Epub 2024 Apr 23. J Physiol. 2024. PMID: 38654581 Free PMC article.
-
Activation of immobility-related hippocampal theta by cholinergic septohippocampal neurons during vestibular stimulation.Hippocampus. 2012 Apr;22(4):914-25. doi: 10.1002/hipo.20955. Epub 2011 May 3. Hippocampus. 2012. PMID: 21542057
-
Hippocampal theta oscillations are slower in humans than in rodents: implications for models of spatial navigation and memory.Philos Trans R Soc Lond B Biol Sci. 2013 Dec 23;369(1635):20130304. doi: 10.1098/rstb.2013.0304. Print 2014 Feb 5. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 24366145 Free PMC article. Review.
-
[Theta rhythm recorded in the hippocampal formation in vitro].Postepy Hig Med Dosw (Online). 2013 Jul 15;67:617-30. doi: 10.5604/17322693.1058537. Postepy Hig Med Dosw (Online). 2013. PMID: 24018425 Review. Polish.
Cited by
-
Pedunculopontine-stimulation obstructs hippocampal theta rhythm and halts movement.Sci Rep. 2025 May 23;15(1):17903. doi: 10.1038/s41598-025-01695-8. Sci Rep. 2025. PMID: 40410186 Free PMC article.
-
Sound Localization of World and Head-Centered Space in Ferrets.J Neurosci. 2022 Jun 1;42(22):4580-4593. doi: 10.1523/JNEUROSCI.0291-22.2022. Epub 2022 May 2. J Neurosci. 2022. PMID: 35501154 Free PMC article.
-
Learning to predict future locations with internally generated theta sequences.PLoS Comput Biol. 2023 May 12;19(5):e1011101. doi: 10.1371/journal.pcbi.1011101. eCollection 2023 May. PLoS Comput Biol. 2023. PMID: 37172053 Free PMC article.
-
Frequency matters: how changes in hippocampal theta frequency can influence temporal coding, anxiety-reduction, and memory.Front Syst Neurosci. 2023 Feb 3;16:998116. doi: 10.3389/fnsys.2022.998116. eCollection 2022. Front Syst Neurosci. 2023. PMID: 36817946 Free PMC article.
-
Acetylcholine modulates the temporal dynamics of human theta oscillations during memory.Nat Commun. 2023 Aug 30;14(1):5283. doi: 10.1038/s41467-023-41025-y. Nat Commun. 2023. PMID: 37648692 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

