Clinical Validation of the Covariates Pharmacokinetic Model for Propofol in an Adult Population
- PMID: 36207643
- PMCID: PMC9700536
- DOI: 10.1007/s40268-022-00404-4
Clinical Validation of the Covariates Pharmacokinetic Model for Propofol in an Adult Population
Abstract
Background and objective: Pharmacokinetic or pharmacokinetic-pharmacodynamic models have been instrumental in facilitating the clinical use of propofol in target-controlled infusion systems in anaesthetic practice. There has been debate over which model should be recommended for practice. The covariates model is an updated pharmacokinetic model for propofol. The aim of this study was to prospectively validate this model in an adult population.
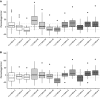
Methods: Twenty-nine patients were included, with a range of ages to assess model performance in younger and older individuals. Subjects received propofol through a target-controlled infusion device programmed with the covariates model. Subjects were randomised to one of two increasing/decreasing regimes of propofol plasma target concentrations between 2 and 5 μg.mL-1. After the start of the infusion, arterial and venous blood samples were drawn at pre-specified timepoints between 1.5 and 20 min and between 1.5 and 45 min, respectively. Predictive performance was assessed using established methodology.
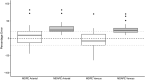
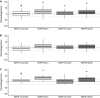
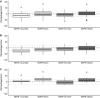
Results: The model achieved a bias of 9 (- 45 to 82) and precision of 24 (9-82) for arterial samples and bias of - 8 (- 64 to 70) and precision of 23 (9-70) for venous samples. Predicted concentrations tended to be higher than the measured concentrations in female individuals but lower in male individuals. There was no clear systematic difference in the bias between younger and older patients.
Conclusions: The covariates propofol pharmacokinetic model achieved an acceptable level of predictive performance, as assessed by both arterial and venous sampling, for use in target-controlled infusion in clinical practice.
Clinical trial registration: NCT01492712 (15 December, 2011).
Plain language summary
Pharmacokinetic models can estimate the changes in the concentration of a drug in the body over time. These have been instrumental in facilitating the clinical use of anaesthetic agents such as propofol in target-controlled infusions, which aim to achieve a set concentration in either plasma or the brain to achieve anaesthesia. The covariates model is a previously described pharmacokinetic model for propofol. The aim of the described study was to validate the performance of the model in an independent adult population. Participants received anaesthesia with propofol through a target-controlled infusion device programmed with the covariates model. The concentration of propofol in the blood was measured at various timepoints and compared to the target concentration specified by the target-controlled infusion device. The analysis showed that overall, the covariates model performed to a level acceptable for use in clinical practice and compared favourably to other pharmacokinetic models.
© 2022. The Author(s).
Conflict of interest statement
Injectomat TIVA Agilia with covariates model was provided by Fresenius Kabi (Brezins, France). SS received consultant honoraria from Fresenius Kabi and lecture honoraria from Aspen Pharmaceuticals. CH received consultant honoraria from Integra LifeSciences and lecture honoraria from AstraZeneca. MS, RC, NS and SM have no conflicts of interest that are directly relevant to the content of this article.
Figures
References
-
- Schnider TW, Minto CF, Gambus PL, et al. The Influence of method of administration and covariates on the pharmacokinetics of propofol in adult volunteers. Anesthesiology. 1998;1(88):1172–1180. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

