Proposed refined diagnostic criteria and classification of eosinophil disorders and related syndromes
- PMID: 36207764
- PMCID: PMC9797433
- DOI: 10.1111/all.15544
Proposed refined diagnostic criteria and classification of eosinophil disorders and related syndromes
Abstract
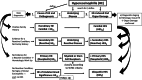
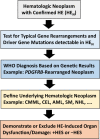
Eosinophilia and eosinophil activation are recurrent features in various reactive states and certain hematologic malignancies. In patients with hypereosinophilia (HE), HE-induced organ damage is often encountered and may lead to the diagnosis of a hypereosinophilic syndrome (HES). A number of known mechanisms and etiologies contribute to the development of HE and HES. Based on these etiologies and the origin of eosinophils, HE and HES are divided into primary forms where eosinophils are clonal cells, reactive forms where an underlying reactive or neoplastic condition is detected and eosinophils are considered to be "non-clonal" cells, and idiopathic HE and HES in which neither a clonal nor a reactive underlying pathology is detected. Since 2012, this classification and the related criteria have been widely accepted and regarded as standard. However, during the past few years, new developments in the field and an increasing number of markers and targets have created a need to update these criteria and the classification of HE and HES. To address this challenge, a Working Conference on eosinophil disorders was organized in 2021. In this conference, a panel of experts representing the relevant fields, including allergy, dermatology, hematology, immunology, laboratory medicine, and pathology, met and discussed new markers and concepts as well as refinements in definitions, criteria and classifications of HE and HES. The outcomes of this conference are presented in this article and should assist in the diagnosis and management of patients with HE and HES in daily practice and in the preparation and conduct of clinical trials.
Keywords: classification; diagnostic criteria; eosinophilic leukemia; hypereosinophilic syndrome; personalized medicine.
© 2022 The Authors. Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no study‐related specific conflicts of interest. The authors declare the following conflicts of interest outside of this project: P.Valent received research grants from Pfizer, BMS/Celgene and AOP Orphan, and consultancy honoraria from Novartis, Pfizer, BMS/Celgene, Blueprint, Accord, and AOP Health. F.R. has received consultancy fees from Astra Zeneca and GlaxoSmithKline (GSK) and receives publication‐related royalty payments from UpToDate contributions. D.S. has been an investigator and/or advisor of AbbVie, AstraZeneca, Galderma, GSK, LEO Pharma, Elli Lilly, Novartis, Pfizer, Sanofi Genzyme. K.M.L. receives publication‐related royalty payments from UpToDate contributions, has a grant from Regeneron, receives royalties from Mayo Foundation, and holds patents for diagnosing, monitoring, and treating eosinophil‐related diseases. J.S. received consultancy honoraria from Novartis, GSK, Astra Zeneca and Blueprint. W.R.S. received consultancy honoraria from AbbVie, BMS/Celgene, Jazz, Novartis, Pfizer, StemLine, and Thermo Fisher. K.S. received consultancy honoraria from Novartis and Blueprint. P.Vandenberghe has received research support from Janssen Biotech and Pfizer via KU Leuven; and honoraria as speaker or advisory board member from Bristol‐Myers‐Squibb, Janssen Biotech, Miltenyi Biotec and Novartis Pharma. G.H. received honoraria from Novartis and Incyte. T.H. is part owner of the Munich Leukemia Laboratory (MLL). T.I.G has been a consultant for Incyte, Blueprint Medicines and Celgene/BMS. C.A. received consultancy honoraria from Novartis and Blueprint Medicines. B.S.B. receives publication‐related royalty payments from Elsevier and UpToDate®/Wolters Kluwer. He receives remuneration for consulting services (Third Harmonic Bio, Acelyrin Inc. and Lupagen) and for serving on the scientific advisory board of Allakos Inc., which he co‐founded, and owns stock in Allakos. He is a co‐inventor on existing Siglec‐8–related patents and thus receives royalty payments from Johns Hopkins University during development and potential sales of such products. The terms of this arrangement are being managed by Johns Hopkins University and Northwestern University in accordance with their conflict of interest policies. A.R. received research funding from Novartis and Blueprint and consultancy honoraria, speaker fees, and travel reimbursement from Novartis, Incyte, GSK, Blueprint, BMS/Celgene and Abbvie. J.G. received research funding from Incyte, Novartis, Kartos, Blueprint Medicines, Deciphera, Cogent Biosciences, Abbvie, Celgene, BMS, Protagonist Therapeutics, and advisory board/consulting honoraria from Incyte, Novartis, Kartos, Blueprint Medicines, Deciphera, Cogent Biosciences, Abbvie, Protagonist Therapeutics, and PharmaEssentia; he is also a PI and receives research funding for the FIGHT‐203 phase II study of pemigatinib in patients with
Figures

References
-
- Gleich GJ. Mechanisms of eosinophil‐associated inflammation. J Allergy Clin Immunol. 2000;105(4):651‐663. - PubMed
-
- Simon D, Simon HU. Eosinophilic disorders. J Allergy Clin Immunol. 2007;119(6):1291‐1300. - PubMed
-
- Curtis C, Ogbogu P. Hypereosinophilic syndrome. Clin Rev Allergy Immunol. 2016;50(2):240‐251. - PubMed
-
- Helbig G, Klion AD. Hypereosinophilic syndromes ‐ an enigmatic group of disorders with an intriguing clinical spectrum and challenging treatment. Blood Rev. 2021;49:100809. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

