Preliminary results from the LUX-Dx insertable cardiac monitor remote programming and performance (LUX-Dx PERFORM) study
- PMID: 36208096
- PMCID: PMC9849434
- DOI: 10.1002/clc.23930
Preliminary results from the LUX-Dx insertable cardiac monitor remote programming and performance (LUX-Dx PERFORM) study
Abstract
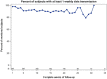
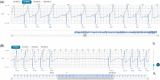
Despite the wide adoption of insertable cardiac monitors (ICMs), high false-positive rates, suboptimal signal quality, limited ability to detect atrial flutter, and lack of remote programming remain challenging. The LUX-Dx PERFORM study was designed to evaluate novel technologies engineered to address these issues. Here, we present preliminary results from the trial focusing on the safety of ICM insertion, remote monitoring rates, and the feasibility of remote programming. LUX-Dx PERFORM is a multicenter, prospective, single-arm, post-market, observational study with planned enrollment of up to 827 patients from 35 sites in North America. A preliminary cohort consisting of the first 369 patients who were enrolled between March and October 2021 was selected for analysis. Three hundred sixty-three (363) patients had ICM insertions across inpatient and outpatient settings. The mean time followed was 103.4 ± 61.8 days per patient. The total infection rate was 0.8% (3/363). Interim results show high levels of remote monitoring with a median 94% of days with data transmission (interquartile range: 82-99). Thirteen (13) in-clinic and 24 remote programming sessions were reported in 34 subjects. Reprogramming examples are presented to highlight signal quality, the ability to detect atrial flutter, and the positive impact of remote programming on patient management. Interim results from LUX-Dx PERFORM study demonstrate the safety of insertion, high data transmission rates, the ability to detect atrial flutter, and the feasibility of remote programming to optimize arrhythmia detection and improve clinical workflow. Future results from LUX-Dx PERFORM will further characterize improvements in signal quality and arrhythmia detection.
Keywords: ICM; arrhythmia; cryptogenic stroke; device programming; remote monitoring.
© 2022 The Authors. Clinical Cardiology published by Wiley Periodicals, LLC.
Conflict of interest statement
Craig Stolen, Brian Kwan, Jonathan Kelly, and David Perschbacher are Employees of Boston Scientific; Jonathan Rosman and John Garner have no conflicts of interest; Harish Manyam is a consultant for Abbott, Boston Scientific, and Jannssen; Mark Richards is the Principal Investigator of the LUX‐Dx PERFORM study and a consultant for Boston Scientific.
Figures

References
-
- Andrade JG, Champagne J, Dubuc M, et al. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: a randomized clinical trial. Circulation. 2019;140:1779‐1788. https://pubmed.ncbi.nlm.nih.gov/31630538/ - PubMed
-
- Sanna T, Diener H‐C, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478‐2486. https://pubmed.ncbi.nlm.nih.gov/24963567/ - PubMed
-
- Edvardsson N, Frykman V, van Mechelen R, et al. Use of an implantable loop recorder to increase the diagnostic yield in unexplained syncope: results from the PICTURE registry. Europace. 2011;13:262‐269. https://pubmed.ncbi.nlm.nih.gov/21097478/ - PMC - PubMed
-
- Afzal MR, Mease J, Koppert T, et al. Incidence of false‐positive transmissions during remote rhythm monitoring with implantable loop recorders. Heart Rhythm. 2020;17:75‐80. https://pubmed.ncbi.nlm.nih.gov/31323348/ - PubMed
-
- Chorin E, Peterson C, Kogan E, et al. Comparison of the effect of atrial fibrillation detection algorithms in patients with cryptogenic stroke using implantable loop recorders. Am J Cardiol. 2020;129:25‐29. https://pubmed.ncbi.nlm.nih.gov/32600783/ - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

